Review
| Rev Diabet Stud,
2013,
10(2-3):121-132 |
DOI 10.1900/RDS.2013.10.121 |
Dyslipidemia and Diabetic Retinopathy
Yo-Chen Chang1, Wen-Chuan Wu2
1Department of Ophthalmology, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
2Department of Ophthalmology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
Address correspondence to: Wen-Chuan Wu, Department of Ophthalmology, Kaohsiung Medical University, 100, Zihyou 1st Rd., San-Ming District, 807, Kaohsiung, Taiwan, e-mail: wcwu.oph@gmail.com
Manuscript submitted May 28, 2013; resubmitted June 23, 2013; accepted June 28, 2013.
Keywords: type 2 diabetes, dyslipidemia, diabetic retinopathy, diabetic macular edema
Abstract
Diabetic retinopathy (DR) is one of the major microvascular complications of diabetes. In developed countries, it is the most common cause of preventable blindness in diabetic adults. Dyslipidemia, a major systemic disorder, is one of the most important risk factors for cardiovascular disease. Patients with diabetes have an increased risk of suffering from dyslipidemia concurrently. The aim of this article is to review the association between diabetic retinopathy (DR) and traditional/nontraditional lipid markers, possible mechanisms involving lipid metabolism and diabetic retinopathy, and the effect of lipid-lowering therapies on diabetic retinopathy. For traditional lipid markers, evidence is available that total cholesterol and low-density lipoprotein cholesterol are associated with the presence of hard exudates in patients with DR. The study of nontraditional lipid markers is advancing only in recently years. The severity of DR is inversely associated with apolipoprotein A1 (ApoA1), whereas ApoB and the ApoB-to-ApoA1 ratio are positively associated with DR. The role of lipid-lowering medication is to work as adjunctive therapy for better control of diabetes-related complications including DR.
Abbreviations: ADVANCE - Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation; AGE - advanced glycation end product; Apo - apolipoprotein; ARICS - Atherosclerosis Risk in Communities Study; BES - Beijing Eye Study; CHS - Cardiovascular Health Study; CSME - clinically significant macular edema; CURES - Chennai Urban Rural Epidemiology Study; DAG - diacylglycerol; DCCT - Diabetes Control and Complications Trial; DME - diabetic macular edema; DR - diabetic retinopathy; ECM - extracellular matrix; ETDRS - Early Treatment Diabetic Retinopathy Study; HbA1c - glycosylated hemoglobin; HDL-C - high-density lipoprotein cholesterol; HMG-CoA - 3-hydroxy-3-methylglutaryl coenzyme A; LDL-C - low-density lipoprotein cholesterol; Lp(a) - lipoprotein(a); MDA - malondialdehyde; MESA - Multi-Ethnic Study of Atherosclerosis study; NCSME - nonclinically significant macular edema; NPDR - nonproliferative DR ; PDR - proliferative DR; PKC - protein kinase C; PPAR - peroxisome proliferators-activated receptor; SMES - Singapore Malay Eye Study; SN-DREAMS - Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study; TG - triglyceride; UKPDS - United Kingdom Prospective Diabetes Study; WESDR - Wisconsin Epidemiologic Study of Diabetic Retinopathy
1. Introduction
Diabetic retinopathy (DR) is a major microvascular complication of diabetes. It is the most common cause of blindness in the working-age population in developed countries [1, 2]. The prevalence of DR increases with duration of diabetes [3]. More than 60% of patients with type 2 diabetes and almost all patients with type 1 have some degree of retinopathy after 20 years' duration of diabetes [3, 4].
DR can be classified into 2 stages:
1. Nonproliferative DR. The fundus findings of nonproliferative DR (NPDR) are microaneurysms, retinal hemorrhage, and capillary nonperfusion (cotton-wool spots, venous beading, and intraretinal microvascular abnormalities).
2. Proliferative DR (PDR) is characterized by the growth of new blood vessels on the surface of the retina. These abnormal vessels bleed easily, resulting in vitreous hemorrhage, subsequent fibrosis, and tractional retinal detachment [5].
Another common diabetes complication is diabetic macular edema (DME), which is also a major cause of vision loss and, which can occur at any stage of DR. DME is characterized by increased vascular permeability and the deposition of hard exudate at the central retina. Retinal hard exudate is thought to be the result of lipoproteins leaking from retinal capillaries into the extracellular space of the retina [6].
Dyslipidemia, a major systemic disorder, is one of the most important risk factors for cardiovascular disease [7, 8]. Although landmark studies have shown that intensive glycemic and blood pressure control can substantially reduce the onset and progression of DR [9, 10], the contribution of lipids to the pathogenesis of DR and DME is not clear. Therefore, in this article, we review relevant articles in a chronological fashion, to clarify the association between dyslipidemia and diabetic retinopathy.
2. Studies on traditional lipid abnormalities and diabetic retinopathy
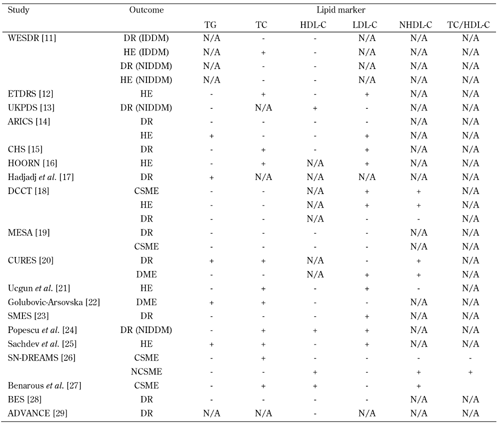
The following subsections provide an overview of the most important clinical studies on diabetic retinopathy (see also Table 1).
Table
1.
Studies on the associations of traditional lipid markers and diabetic retinopathy |
|
 |
 |
Legend:
ADVANCE – Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation. ARICS – Atherosclerosis Risk in Communities Study, BES – Beijing Eye Study, CHS – Cardiovascular Health Study, CSME – clinically significant macular edema, CURES – Chennai Urban Rural Epidemiology Study, DCCT – Diabetes Control and Complications Trial, DR – diabetic retinopathy, ETDRS – Early Treatment Diabetic Retinopathy Study, HDL-C – high-density lipoprotein cholesterol, HE – hemorrhage edema, IDDM – insulin-dependent diabetes mellitus, LDL-C – low-density lipoprotein cholesterol, MESA – Multi-Ethnic Study of Atherosclerosis study, N/A – not available, NHDL-C – non-HDL- cholesterol, NIDDM – non-insulin-dependent diabetes mellitus, SMES – Singapore Malay Eye Study, SN-DREAMS – Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study, TC – total cholesterol, TG – triglceride, UKPDS – United Kingdom Prospective Diabetes Study, WESDR – Wisconsin Epidemiologic Study of Diabetic Retinopathy. References [11-29]. |
|
2.1 The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) XIII
The purpose of the WESDR XIII study was to elucidate the relationship of serum cholesterol to retinopathy and hard exudate [11]. Serum total and high-density lipoprotein cholesterol (HDL-C) were measured between 1984 and 1986. There was a significant trend towards an association between increasing severity of diabetic retinopathy and of retinal hard exudate and increasing cholesterol in insulin-dependent persons. Cholesterol levels were not related to the severity of either ocular condition in older-onset patients. HDL-C was unrelated to the severity of either lesion. Multiple logistic regression analyses did not indicate that cholesterol was a significant factor in describing the severity of retinopathy in any group, but did suggest that it was a significant factor in describing the severity of retinal hard exudate.
2.2 The Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22
In 1996, the ETDRS published a report to evaluate the relationship between serum lipid levels and retinal hard exudates in patients with diabetic retinopathy [12]. This study included 2709 patients whose serum lipid levels were measured. At baseline, the patients who had elevated serum total cholesterol or elevated serum low-density lipoprotein cholesterol (LDL-C) were more likely to have retinal hard exudate.
2.3 The United Kingdom Prospective Diabetes Study (UKPDS)
UKPDS is a multi-center, randomized, controlled clinical study of therapy in patients with NIDDM [13]. The study enrolled 2964 Caucasian patients who had both eyes photographed. Interestingly, univariate analysis revealed that higher high-density lipoprotein cholesterol levels were also associated with more severe retinopathy. However, the authors offered no explanation for this finding. In addition, triglyceride levels and low-density lipoprotein cholesterol levels did not appear to be related to the severity of retinopathy.
2.4 The Atherosclerosis Risk in Communities Study (ARICS)
The purpose of the ARICS study was to describe the prevalence of retinopathy and its associations with atherosclerosis and vascular risk factors in people with diabetes [14]. In this cross-sectional study, the authors enrolled patients with diabetes living in four United States communities. Retinopathy was detected in 328/1600 (20.5%) of those with diabetes; 6.6% had hard exudate, 1.8% had proliferative diabetic retinopathy, and 1.6% had macular edema. The presence of retinal hard exudates was associated with plasma LDL-C and plasma lipids.
2.5 The Cardiovascular Health Study (CHS)
The CHS was a population-based cohort study, aimed at the analysis and description of the association of retinopathy with atherosclerosis and atherosclerotic risk factors in patients with diabetes [15]. Univariate analysis showed that retinopathy was associated with higher average systolic blood pressure, higher plasma total and LDL cholesterol, and the presence of cardiovascular disease. Retinopathy was not associated with plasma HDL-C and triglycerides. Univariate analysis also indicated that both mean plasma total cholesterol and LDL-C were higher in patients with retinal hard exudate than in patients without this disease.
2.6 The Hoorn Study
The purpose of the population-based Hoorn Study was to describe the risk factors for retinopathy in diabetic and nondiabetic individuals (2,484 Caucasians aged from 50 to 74 years) [16]. The prevalence of retinopathy was positively associated with elevated blood pressure, BMI, serum cholesterol and triglyceride levels in all glucose categories. In addition, elevated blood pressure and plasma total and LDL cholesterol levels showed associations with retinal hard exudate.
2.7 A study from France
A prospective study from France aimed to describe the influence of serum lipids on the development and progression of microvascular complications in 297 patients with type 1 diabetes without end-stage renal disease [17]. Both in the whole cohort and at baseline, serum triglyceride levels were higher in patients with progressive nephropathy and retinal events than in those without. After adjustment for systolic blood pressure, diabetes duration, gender, stage of complications at baseline, and HbA1c, the relative risk for progression was 2.01 for nephropathy and 2.30 for retinopathy in patients with serum TG in the highest tertile. Based on the above study, the authors concluded that high triglyceride levels were an independent predictive factor of both renal and retinal complications in patients with type 1 diabetes.
2.8 Results of the Diabetes Control and Complications Trial (DCCT)
The DCCT study evaluated the relationship between serum lipid levels and clinically significant macular edema (CSME), hard exudate, and other DR end points in 1441 patients with type 1 diabetes [18]. The authors found that total-to-HDL cholesterol ratio and LDL-C predicted development of CSME and hard exudate. Higher serum lipids were associated with increased risk of CSME and retinal hard exudate. The authors concluded that lipid-lowering treatment among type 1 diabetic subjects may also decrease the risk of CSME, an important cause of vision loss.
2.9 The Multi-Ethnic Study of Atherosclerosis (MESA)
The purpose of the cross-sectional MESA study was to describe the risk factors for diabetic retinopathy in a multi-ethnic US population of whites, blacks, Hispanics, and Chinese [19]. The authors found that DR, CSME, or vision-threatening retinopathy were not significantly associated with HDL-C, LDL-C, and TG in 778 individuals aged from 45 to 85 years with diabetes.
2.10 Results of the Chennai Urban Rural Epidemiology Study (CURES), Eye Study 2
The CURES study evaluated the association of serum lipids with DR in 1736 patients with type 2 diabetes [20]. The authors found that mean serum cholesterol, serum TG and HDL-C concentrations were significantly higher in subjects with DR compared with those without DR. Multiple logistic regression analysis revealed that total cholesterol, non-HDL-C and serum TG were associated with DR and DME was associated with non-HDL-C and LDL-C.
2.11 A study from Turkey
Ucgun et al. conducted a small clinical study to evaluate the relationship between serum lipid levels and exudative diabetic maculopathy in 54 patients with nonproliferative diabetic retinopathy [21]. Twenty-seven patients with exudative diabetic macular edema were included in group A and 27 patients without exudative diabetic macular edema were included in group B. Serum levels of cholesterol (p = 0.038) and LDL-C (p = 0.026) were significantly higher in patients with exudative diabetic macular edema. However, TG, HDL-C, and VLDL-C levels did not differ between the two groups.
2.12 A study from Macedonia
The aim of this study was to underline the role of elevated serum lipids in the onset of macular edema and hard exudates in patients with type 2 diabetes [22]. The diabetic patients that manifested diabetic maculopathy had significantly higher levels of total lipids, TG, total cholesterol, and cholesterol ester as compared to those without diabetic maculopathy. Although values for HDL-C and LDL-C were higher in patients with diabetic maculopathy, there were no statistically significant differences.
2.13 Results from the Singapore Malay Eye Study (SMES)
The purpose of this population-based cross-sectional study was to describe the prevalence and risk factors for diabetic retinopathy in Asian Malays [23]. In persons with diabetes, the overall prevalence of any retinopathy was 35.0%. Multiple logistic regression analysis revealed that LDL-C is an independent risk factor for any retinopathy.
2.14 A study from Romania
This study investigated the association between DR, lipid disorder,, and blood pressure in subjects with type 2 diabetes without known cardiovascular diseases [24]. The authors examined 100 patients with type 2 diabetes but without clinical evidence of coronary, cerebrovascular or peripheral artery disease. The patients who presented with diabetic retinopathy had significantly higher values for total cholesterol and LDL-C and lower HDL-C levels compared to patients without retinopathy.
2.15 A study from North India
The purpose of this observational case-control study was to describe the association of various systemic risk factors with retinal hard exudates in type 2 diabetic North Indian patients of NPDR with CSME and to measure the incidence of dyslipidemia in these patients [25]. On univariate analysis, retinal hard exudates were significantly associated with systolic blood pressure, serum cholesterol, serum LDL-C, and serum TG levels. On linear regression analysis, however, serum cholesterol and serum LDL-C were found to be independent risk factors affecting the density of retinal hard exudate.
2.16 SN-DREAMS Report Number 13
This population-based cross-sectional study estimated the prevalence of DME, both CSME and NCSME, and reported the association of the latter with dyslipidemia [26]. Prevalence was 31.76% for overall diabetic macular edema, 25.49% for NCSME, and 6.27% for CSME. Logistic regression multivariate analysis revealed that high serum LDL-C, high serum non-HDL-C, and a high cholesterol ratio related to NCSME, while only high serum total cholesterol related to CSME.
2.17 A study from Australia
This study assessed the association of serum lipids with DR, DME, and macular thickness in 500 adults with diabetes. DR, DME, and CSME were present in 321 (66.2%), 149 (33.0%), and 68 (15.0%) patients, respectively [27]. In multivariate analysis adjusted for traditional risk factors and lipid medications, patients with higher total cholesterol, LDL-C, and non-HDL-C were more likely to have CSME. No association was found for serum lipids with macular thickness, as assessed by OCT. Based on the above findings, the authors concluded that serum lipids are independently associated with CSME but not with DR, mild or moderate DME, or macular thickness.
2.18 The Beijing Eye Study (BES)
The purpose of this population-based study (2945 subjects) was to determine associations between dyslipidemia and ocular diseases [28]. By multivariable regression analysis, dyslipidemia was significantly associated with higher intraocular pressure and beta zone of parapapillary atrophy. Dyslipidemia was not significantly associated with the prevalence of glaucoma, retinal vein occlusions, DR, presence of retinal vascular abnormalities such as focal or general arteriolar narrowing, and age-related macular degeneration.
2.19 The ADVANCE Study
This study examined the association between HDL-C and microvascular (renal and retinal) disease in a cohort of 11,140 patients with type 2 diabetes [29]. During follow-up, 32% of the patients developed new or worsening microvascular disease, with 28% experiencing a renal event and 6% a retinal event. In this study, the authors concluded that, in patients with type 2 diabetes, HDL-C level is an independent risk factor for the development of microvascular disease affecting the kidney but not the retina.
Even though numerous studies have explored the associations between DR and lipid abnormalities, the results obtained remain inconsistent in contrast to other definite risk factors for DR such as blood sugar and blood pressure control. In TG, only 2 studies show an association with DR [17, 20] and 3 studies show an association with retinal hard exudates [14, 25] or DME [22]. A significant association between total-C and DR was found in 3 studies [15, 20, 24] and 8 studies revealed a significant association between total-C and retinal hard exudates[11, 12, 16, 21, 25] or DME [22, 26, 27]. With regard to LDL-C, there are 3 studies [15, 23, 24] showing a significant association with DR and 8 studies demonstrating an association with DME [18, 20] or retinal hard exudates [12, 14, 16, 18, 21, 25]. There is no single lipid measure consistently found to be associated with DR. However, more evidence has been obtained that links total-C or LDL-C with the presence of hard exudates [11, 12, 14, 16, 18, 21] since retinal exudates are often due to leakage of lipid from abnormal retinal capillaries and are usually associated with DME.
3. Possible mechanisms involving lipid and DR
Hyperglycemia has been shown to cause cell damage through the following pathways: the polypol pathway, overactivity of the hexosamine pathway, advanced glycation end product (AGE) formation with increased expression of AGE receptors, and activation of protein kinase C (PKC) isoforms [30]. However, the mechanism for the associations between traditional lipid markers and DR remains unclear. Of the hyperglycemia-associated pathways mentioned above, the PKC and AGE pathways interact with lipid levels. Protein kinase C (PKC) is a family of 10 enzymes, in which the 1/2 isoform appears to be closely associated with the development of DR [31]. Hyperglycemia leads to an increase in glucose flux through the glycolysis pathway, which in turn increases de novo synthesis of diacylglycerol (DAG), the key activator of PKC in physiology [32]. In addition, the accumulation of long-chain FAs are simultaneously converted into DAG. The expression of the PKC 1/2 isoform is enhanced in patients with diabetes. Since PKC is involved in a number of physiological processes, its upregulation contributes to the pathogenesis of DR in the form of differential synthesis of extracellular matrix (ECM) proteins and ECM remodeling, enhanced release of angiogenic factors, endothelial and leukocyte dysfunction leading to capillary occlusion and leukostasis, and changes in blood flow to the retina [33].
AGEs are generated from nonenzymatic reactions between reducing sugars and lipoproteins [34]. AGEs form at a constant but slow rate in the normal body starting at embryonic development and accumulating over time. However, their formation is markedly accelerated in diabetes because of the increased availability of glucose [35]. In a highly oxidative environment such as the retina, the accumulation of lipid and modification of protein will cause an accumulation of lipoxidation end products (ALEs).
There are two kinds of AGEs associated with DR pathogenesis: carboxyethylpyrrole [36] and malondialdehyde (MDA) [37]. AGEs are important pathogenic mediators of almost all diabetic complications. They are found, for example in the retinal vessels of diabetic patients, and their levels correlate with those in the serum as well as with the severity of the retinopathy. The interaction of AGEs with specific cell surface receptors has been implicated in the development of DR. These AGE receptors include the RAGE, galectin-3, CD36, and the macrophage scavenger receptor [38]. There is evidence from animal studies that exposure to high levels of AGEs contributes to renal and vascular complications. In a study by Hammes et al., the retinal capillaries showed an increased accumulation of AGEs and loss of pericytes 26 weeks after the development of diabetes in rats. Treatment with aminoguanidine (pimagedine) hydrochloride, an AGE formation inhibitor, significantly reduced AGE accumulation and prevented the formation of microaneurysms, acellular capillaries, and pericyte loss [39].
4. Associations with nontraditional lipid markers
In conventional lipid profiles, the plasma concentrations of each class are expressed in terms of its contribution to total cholesterol, providing only a crude description of what is a very complex system. Modifications of lipoproteins by glycation and oxidation and/or variations in the size (i.e., diameter) distributions of lipoprotein particles within the major lipoprotein classes are not reflected in conventional profiles. A new technique, nuclear magnetic resonance (NMR) analysis of whole serum, can rapidly determine concentrations of 15 different lipoprotein subclasses, designated according to particle size, without physical separation of the subclasses [40]. In cardiovascular diseases, lipoprotein(a) (Lp(a)) has been found to be strongly associated with stroke and coronary heart disease [41, 42].
Studies investigating the association between Lp(a) and DR have yielded conflicting results to date. Maioli et al. enrolled two groups of patients with type 1 diabetes of at least 15 years duration: 25 patients with active retinopathy and 27 patients without clinically detectable retinal lesions. Thirty-eight healthy subjects of the same age and sex served as controls. The authors found that serum Lp(a) was significantly higher in the patients with active retinopathy than in those without clinically detectable retinal lesions or the control subjects [43]. Suehiro et al. enrolled 412 patients with type 2 diabetes in a similar study. Serum Lp(a) concentrations were significantly higher in diabetic patients than in normal controls. Furthermore, the patients with DR, especially proliferative retinopathy, showed higher serum Lp(a) concentrations than those without DR [44]. In contrast, there are some reports which show negative associations [45-47].
In addition to Lp(a), serum apolipoproteins (Apo) profiles have been investigated for an association with DR. The two major lipoprotein classes, LDL-C and HDL-C, have their own unique structures and functions. LDL-C particles contain apolipoprotein B (ApoB) and deliver cholesterol to tissues. HDL-C particles contain apolipoprotein A (ApoA), apolipoprotein C (ApoC), and apolipoprotein E (apoE), and remove cholesterol from tissues. ApoA1 is the major ApoA protein accounting for about 70% of the total HDL protein mass [48]. Sinav et al. enrolled 78 patients with type 1 diabetes in a study of plasma lipids and lipoproteins in type 1 diabetic patients with retinopathy [49]. It was found that the changes in plasma TG, HDL phospholipid, and Apo A and B, were not significantly associated with the development of DR. Kawai et al. examined the Apo concentrations in reflex tears from healthy and type 1 diabetes subjects and correlated them with the stage of DR [50]. The ApoA1 concentrations in the tears obtained from type 1 diabetes patients with retinopathy were significantly higher than those from patients with no or negligible retinopathy, and ApoA1 was not detected in healthy subjects by Western blotting. Therefore, the authors concluded that there was increased secretion of native ApoA1 from the main lacrimal gland in patients with advanced DR.
In Mexico, Santos et al. enrolled 36 patients with DR and 22 unrelated and apparently healthy age-matched individuals to determine the relationship between ApoE polymorphism and the severity of retinal hard exudate in Mexican patients with NIDDM [51]. The frequency of severe retinal hard exudate was higher in the epsilon4 allele carriers. Their results suggest that the epsilon4 allele of the ApoE gene is a potential risk factor for the severity of retinal hard exudate and visual loss in type 2 diabetic Mexican patients with DR. In contrast, the study by Liew et al. showed that ApoE gene polymorphisms were not associated with DR in either Caucasians or African-Americans with type 2 diabetes [52]. Klein et al. studied 409 patients with type 1 diabetes in the DCCT/EDIC cohort to investigate the associations of ApoC3 protein and ApoC3 gene variation with microvascular disease complications in type 1 diabetes [53]. The ApoC3 concentration was significantly higher in the group of patients with severe retinopathy compared to those with moderate or mild retinopathy.
Simo et al. studied vitreous samples from 4 diabetic patients with PDR and 8 nondiabetic patients with macular hole for proteomic analysis of ApoA1 and ApoH [54]. The authors found that intravitreous ApoA1 and ApoH levels were significantly higher in patients with PDR than in the control group. In addition, the ApoA1 and ApoH mRNA levels obtained from the retinas of diabetic donors were significantly higher than those obtained from nondiabetic donors. Retinal pigment epithelium was the main contributor to the differences. Recently, Sasongko et al. conducted a cross-sectional study of 224 diabetic patients to compare the associations of serum Apos with DR [55]. In this study, ApoA1 levels were inversely associated with DR, whereas ApoB and the ApoB-to-ApoA1 ratio were positively associated with diabetic retinopathy. ApoA1 and ApoB and the ApoB-to-ApoA1 ratio were significantly and independently associated with DR and DR severity and improved the ability to discriminate DR by 8%.
In a Chinese population, Hu et al. collected serum samples from 25 type 2 diabetic patients with very mild NPDR and 25 type 2 diabetic patients with PDR [56]. They found that there were significant associations between the decreased ApoA1 and low ApoA1/ApoB ratio in serum and PDR. Their findings were consistent with the results obtained by Sasongko et al. [55]. The findings from these two study groups are very encouraging. The beneficial associations of ApoA1 and deteriorating associations of ApoB/A1 with microvascular function seen in this study may be similar to findings in larger vessels [57, 58]. ApoA1, which is the structural protein of HDL-C, can promote vasoprotective mechanisms via its ability to promote reverse cholesterol transport from peripheral tissue to the liver and to inhibit LDL-C from oxidation, which may induce smooth muscle cell cytotoxicity and vascular endothelial dysfunction [59]. In the retina, ApoA1 is proposed as a key factor for preventing lipid accumulation [60] and a potent scavenger of oxygen-reactive species for protecting the retina from the oxidative stress caused by diabetes [61]. In contrast, ApoB is the main component of LDL-C and is a reflection of atherogenicity [62]. Low ApoA1/ApoB ratio in serum is considered to be a risk for atherosclerosis [63]. Therefore, ApoB/A1 levels may reflect both damaging and protective lipoprotein pathways [62, 64]. However, the sample size in these two studies was small and their findings need to be reproduced in larger longitudinal studies.
5. Lipid-lowering therapy and DR
Currently, lipid-lowering agents are recommended for patients with dyslipidemia, including those with diabetes. The recommendations are based on the reduction of cardiovascular morbidity by lowering lipid levels [65, 66]. Several lipid-lowering drugs have been under investigation for a possible protective role in DR.
5.1 Fibrates
Fenofibrate is a peroxisome proliferator-activated receptor (PPAR) agonist indicated for the treatment of hypertriglyceridemia and mixed dyslipidemia. Its main action is to lower plasma TG levels, but it also reduces total and LDL-C, raises HDL-C, and decreases concentration of small LDL-C particles and ApoB. Three randomized studies were conducted with clofibrate alone or combined with androsterone. They showed beneficial effects on retinal and macular hard exudates. However, the improvement in retinal hard exudates was not associated with any significant improvement in visual acuity or reduction in retinal hemorrhages [67-69]. In a pilot study of 11 subjects with hyperlipoproteinemia and mild to moderate background DR treated with etofibrate for a period of 6 months, regression of hard exudates was observed in 7 out of 10 patients [70]. In a larger double-blind study, etofibrate or a placebo was given for one year to 296 subjects with DR [71]. A significant improvement in retinal photograph grading (by consensus of 3 experts) was demonstrated in 46% and 32% of subjects with etofibrate and placebo, respectively, but no significant change in visual acuity was achieved. The first study with fenofibrate was conducted in 51 patients with hyperlipidemia and diabetic exudative retinopathy, treated for at least one year [72]. The decrease in total cholesterol and LDL-C was associated with regression of hard retina exudates.
An important study was recently conducted regarding the effect of fenofibrate on patients with dyslipidemia and DR. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study [73] aimed to assess whether long-term lipid-lowering therapy with fenofibrate could reduce the progression of retinopathy and the need for laser treatment in patients with type 2 diabetes. This multinational randomized trial consisted of 9795 patients aged 50-75 years with type 2 diabetes. Eligible patients were randomly assigned to receive fenofibrate 200 mg/day (n = 4895) or matching placebo (n = 4900). In this study, the requirement for first laser treatment for all retinopathy was significantly lower in the fenofibrate group than in the placebo group (164 patients on fenofibrate (3.4%) vs. 238 on placebo (4.9%)). Over 5 years, patients treated with fenofibrate were less likely to demonstrate progression of preexisting retinopathy or to develop macular edema. A substudy conducted in 1012 patients explored effects on retinopathy outcomes in the FIELD study in more detail. In this ophthalmological substudy, retinopathy status and severity were assessed from color fundus photographs of the macula and a disc/nasal field taken at baseline at 2 years and 5 years and graded by applying Early Treatment Diabetic Retinopathy Study (ETDRS) criteria. A marked and significant reduction in the risk of laser treatment for retinopathy was again demonstrated for fenofibrate vs. placebo [73]. Interestingly, this effect seemed unrelated to decreased serum levels of TG and cholesterol. It was, probably achieved by inhibiting vascular endothelial growth factor (VEGF) to decrease neovascularization and inflammation.
5.2 Statins
Statin is an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. The benefits of statin treatment in the primary and secondary prevention of cardiovascular disease have been unequivocally demonstrated [75-76]. These medications may be effective in patients with diabetes because the atherogenicity of LDL-C particles is increased in diabetes. In a pilot study, Gordon et al. [76] studied the effect of pravastatin, an inhibitor of HMG-CoA reductase, in six patients with diabetes with nonproliferative diabetic retinopathy and found the drug to be beneficial in improving retinopathy and reducing hard exudates. In a more recent study, another HMG-CoA reductase inhibitor, simvastatin, was found to retard the progression of retinopathy in patients with diabetes with hypercholesterolemia [77].
Two small randomized studies were performed suggesting a curative effect of the two frequently used statins: simvastatin and atorvastatin. In a 6-month placebo-controlled study in 50 patients with non-clinically significant macular edema, visual acuity improved in 16% of the patients in the simvastatin group and worsened in 28% of the placebo group [77]. In another study, atorvastatin was given for 4 months after laser treatment for clinically significant macular edema. The extension of edema into the central retinal area was absent in the atorvastatin group and occurred in 25% of the control group [78].
The primary prevention Collaborative Atorvastatin Diabetes Study (CARDS), which included 2838 patients over a median follow-up of 3.9 years, showed that 10 mg atorvastatin daily resulted in a trend to reduction of laser therapy compared with placebo, but there was no influence on diabetic retinopathy progression [79]. In a recent case-control study, the development of diabetic retinopathy over 5 years in 114 patients was not influenced by the use of a statin [80]. Thus, the influence of statins on diabetic retinopathy continues to be debated. Better evidence on the effects of larger doses of statins is required. If there is an effect, it is likely to be small.
The results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study were published in 2010. There were 10,251 participants with type 2 diabetes enrolled in this randomized trial. In the lipid arm of the ACCORD study, 5518 patients with dyslipidemia were randomly assigned in a 2-by-2 factorial design to receive simvastatin in combination with either fenofibrate or matching placebo. At 4 years, the rates of progression of diabetic retinopathy were 7.3% with intensive glycemia treatment (HbA1c < 6.0%), versus 10.4% with standard therapy (HbA1c level, 7.0 to 7.9%, p = 0.003); 6.5% with fenofibrate for intensive dyslipidemia therapy, versus 10.2% with a placebo (p = 0.006); and 10.4% with intensive blood-pressure therapy, versus 8.8% with standard therapy (p = 0.29) [81]. In summary, at the end of the ACCORD study the rates of progression of diabetic retinopathy were significantly reduced in the intensive glycemic control group and in the fenofibrate group, but not in the intensive blood pressure control group. The report on the Steno type 2 randomized study revealed a significant reduction in the number of patients with progression in retinopathy with intensive therapy (treated with statins and/or fibrates to achieve TG < 1.7 mmol/l, total-C < 5.0 mmol/l, and HDL-C > 1.1 mmol/l) compared with standard therapy (treated with statins and/or fibrates to attain TG < 2.2 mmol/l, total-C < 6.5 mmol/l, and HDL-C > 0.9 mmol/l). However, no significant difference in visual acuity was found between the groups [82].
Based on the above studies, lipid-lowering medications may work as adjunctive therapy to provide better control of DR than the traditional concept of tight control of blood sugar and blood pressure and laser treatment. More experimental studies are necessary to understand the mechanisms associated with macular edema, its formation and its consequences on retinal neurons metabolism and function.
6. Conclusions
Improvements in diabetes care and management are crucial to decrease the incidence and severity of DR. Nevertheless, DR remains the most common cause of legal blindness in adults in developed countries. In spite of the lack of definite associations between traditional lipid markers and DR, lipid-lowering therapy may be an effective adjunctive agent for DR, particularly for patients with DME requiring laser treatment. Optimizing the medical management of diabetic retinopathy should address the control of glycemia, blood pressure, and lipids, and, based on recent trials, specific therapies using fenofibrate with a statin and candesartan should be considered. In addition, a nontraditional lipid marker such as Apo might be a candidate for better prediction of DR severity than traditional lipid markers. However, further large-scale studies are necessary to elucidate the mechanisms of these associations and the mechanisms by which lipid lowering therapy exerts its reported benefits.
Disclosures: The authors report no conflict of interests.
References
- Cheung N, Mitchell P, Wong YT. Diabetic retinopathy. Lancet 2010. 376(9735):124-136. [DOD] [CrossRef]
- Klein R, Klein BE. Are individuals with diabetes seeing better?-a long-term epidemiological perspective. Diabetes 2010. 59(8):1853-1860. [DOD] [CrossRef]
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998. 105(10):1801-1815. [DOD] [CrossRef]
- Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006. 141(3):446-455. [DOD] [CrossRef]
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy. A systemic review. JAMA 2007. 298(8):902-916. [DOD] [CrossRef]
- Toussaint D, Cogan D, Kuwabara T. Extravascular lesions of diabetic retinopathy. Arch Ophthalmol 1962. 67(1):42-47. [DOD] [CrossRef]
- Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000. 284(3):311-318. [DOD] [CrossRef]
- Rodgers A, MacMahon S, Yee T, Clark T. For the Eastern Stroke and Coronary Heart Disease Collaboration Research Group. Blood pressure, cholesterol, and stroke in eastern Asia. Lancet 1998. 352(9143):1801-1807. [DOD] [CrossRef]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993. 329(14):977-986. [DOD] [CrossRef]
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM, UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004. 122(11):1631-1640. [DOD] [CrossRef]
- Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology 1991. 98(8):1261-1265. [DOD] [CrossRef]
- Chew EY, Klein ML, Ferris FL 3rd, Remaley NA, Murphy RP, Chantry K, Hoogwerf BJ, Miller D. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 1996. 114(9):1079-1084. [DOD] [CrossRef]
- Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, Turner RC, United Kingdom Prospective Diabetes Study 30. Diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol 1998. 116(3):297-303. [DOD] [CrossRef]
- Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, Brancati FL, Hubbard LD, Couper D, ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the Atherosclerosis Risk in Communities study. Ophthalmology 2002. 109(7):1225-1234. [DOD] [CrossRef]
- Klein R, Marino EK, Kuller LH, Polak JF, Tracy RP, Gottdiener JS, Burke GL, Hubbard LD, Boineau R. The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. Br J Ophthalmol 2002. 86(1):84-90. [DOD] [CrossRef]
- van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Blood pressure, lipids, and obesity are associated with retinopathy: the Hoorn study. Diabetes Care 2002. 25(8):1320-1325. [DOD] [CrossRef]
- Hadjadj S, Duly-Bouhanick B, Bekherraz A, BrIdoux F, Gallois Y, Mauco G, Ebran J, Marre M. Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes Metab 2004. 30(1):43-51. [DOD] [CrossRef]
- Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes 2004. 53(11):2883-2892. [DOD] [CrossRef]
- Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006. 141(3):446-455. [DOD] [CrossRef]
- Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians - the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-2. Diabet Med 2006. 23(9):1029-1036. [DOD] [CrossRef]
- Ucgun NI, Yildirim Z, Kilic N, Gürsel E. The importance of serum lipids in exudative diabetic macular edema in type 2 diabetic patients. Ann N Y Acad Sci 2007. 1100:213-217. [DOD] [CrossRef]
- Golubovic-Arsovska M. Association of dyslipidaemia with macular oedema and hard exudates in diabetic maculopathy. Prilozi 2007. 28(2):149-160. [DOD]
- Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, Lim SC, Tai ES, Mitchell P. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology 2008. 115(11):1869-1875. [DOD] [CrossRef]
- Popescu T, Mota M. Dyslipidemia and hypertension in patients with type 2 diabetes and retinopathy. Rom J Intern Med 2009. 47(3):235-241. [DOD]
- Sachdev N, Sahni A. Association of systemic risk factors with the severity of retinal hard exudates in a north Indian population with type 2 diabetes. J Postgrad Med 2010. 56(1):3-6. [DOD] [CrossRef]
- Raman R, Rani PK, Kulothungan V, Rachepalle SR, Kumaramanickavel G, Sharma T. Influence of serum lipids on clinically significant versus nonclinically significant macular edema: SN-DREAMS Report number 13. Ophthalmology 2010. 117(4):766-772. [DOD] [CrossRef]
- Benarous R, Sasongko MB, Qureshi S, Fenwick E, Dirani M, Wong TY, Lamoureux EL. Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Invest Ophthalmol Vis Sci 2011. 52(10):7464-7469. [DOD] [CrossRef]
- Wang S, Xu L, Jonas JB, You QS, Wang YX, Yang H. Dyslipidemia and eye diseases in the adult Chinese population: the Beijing eye study. Plos One 2012. 7(3):e26871. [DOD] [CrossRef]
- Morton J, Zoungas S, Li Q, Patel AA, Chalmers J, Woodward M, Celermajer DS, Beulens JW, Stolk RP, Glasziou P, et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care 2012. 35(11):2201-2206. [DOD] [CrossRef]
- Lim LS, Wong TY. Lipids and diabetic retinopathy. Expert Opin Biol Ther 2012. 12(1):93-105. [DOD] [CrossRef]
- Koya D and King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998. 47(6):859-866. [DOD] [CrossRef]
- Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci 2006. 27(6):317-323. [DOD] [CrossRef]
- Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol 1997. 273:H2442-H2451. [DOD]
- Stitt AW. AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci 2010. 51(10):4867-4874. [DOD] [CrossRef]
- Peppa M, Uribarri J, Vlassara H. Advanced glycation end products, and diabetes complications: what is new and what works. Clinical Diabetes 2003. 21(4):186-187. [DOD] [CrossRef]
- Fathallah L, Obrosova IG. Increased retinal lipid peroxidation in early diabetes is not associated with ascorbate depletion or changes in ascorbate redox state. Exp Eye Res 2001. 72:719-723. [DOD] [CrossRef]
- Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem 2003. 278(43):42027-42035. [DOD] [CrossRef]
- Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Current Diabetes Reports 2011. 11(4):244-252. [DOD] [CrossRef]
- Hammes H P, Martin S, Federlin K, Geisen K, Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci USA 1992. 88(24):11555-11558. [DOD] [CrossRef]
- Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab 2002. 48(3-4):171-180. [DOD]
- Smolders B, Lemmers R, Thijs V. Lipoprotein(a) and stroke: a meta-analysis of observational studies. Stroke 2007. 38(6):1959-1966. [DOD] [CrossRef]
- Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000. 102(10):1082-1085. [DOD] [CrossRef]
- Maioli M, Tonolo G, Pacifico A, Ciccarese M, Brizzi P, Kohner EM, Porta M. Raised serum apolipoprotein (a) in active diabetic retinopathy. Diabetologia 1993. 36(1):88-90. [DOD] [CrossRef]
- Suehiro M, Ohkubo K, Kato H, Kido Y, Anzai K, Oshima K, Ono J. Analyses of serum lipoprotein(a) and the relation to phenotypes and genotypes of apolipoprotein(a) in type 2 diabetic patients with retinopathy. Exp Clin Endocrinol Diabetes 2002. 110(7):319-324. [DOD] [CrossRef]
- Winocour PH, Bhatnagar D, Ishola M, Arrol S, Durrington PN. Lipoprotein (a) and microvascular disease in type 1 (insulin-dependent) diabetes. Diabet Med 1991. 8(10):922-927. [DOD] [CrossRef]
- Boemi M, Sirolla C, Amadio L, Fumelli P, James RW. Lipoprotein(a) and retinopathy in IDDM and NIDDM patients. Diabetes Care 1997. 20(1):115. [DOD]
- Haffner SM, Klein BE, Moss SE, Klein R. Lp(a) is not related to retinopathy in diabetic subjects. Eur J Ophthalmol 1995. 5(2):119-123. [DOD]
- Schultz JR, Verstuyft JG, Gong EL, Nichols AV, Rubin EM. Protein composition determines the anti-atherogenic properties of HDL in transgenic mice. Nature 1993. 365(6448):762-764. [DOD] [CrossRef]
- Sinav S, Onelge MA, Onelge S, Sinav B. Plasma lipids and lipoproteins in retinopathy of type I (insulin-dependent) diabetic patients. Ann Ophthalmol 1993. 25(2):64-66. [DOD]
- Kawai S, Nakajima T, Hokari S, Komoda T, Kawai K. Apolipoprotein A-I concentration in tears in diabetic retinopathy. Ann Clin Biochem 2002. 39(1):56-61. [DOD] [CrossRef]
- Santos A, Salguero ML, Gurrola C, Munoz F, Roig-Melo E, Panduro A. The epsilon4 allele of apolipoprotein E gene is a potential risk factor for the severity of macular edema in type 2 diabetic Mexican patients. Ophthalmic Genet 2002. 23(1):13-19. [DOD] [CrossRef]
- Liew G, Shankar A, Wang JJ, Klein R, Bray MS, Couper DJ, Wong TY. Apolipoprotein E gene polymorphisms are not associated with diabetic retinopathy: the atherosclerosis risk in communities study. Am J Ophthalmol 2006. 142(1):105-111. [DOD] [CrossRef]
- Klein RL, McHenry MB, Lok KH, Hunter SJ, Le NA, Jenkins AJ, Zheng D, Semler A, Page G, Brown WV, et al. Apolipoprotein C-III protein concentrations and gene polymorphisms in Type 1 diabetes: associations with microvascular disease complications in the DCCT/EDIC cohort. J Diabetes Complications 2005. 19(1):18-25. [DOD] [CrossRef]
- Simo R, Higuera M, Garcia-Ramirez M, Canals F, Garcia-Arumi J, Hernandez C. Elevation of apolipoprotein A-I and apolipoprotein H levels in the vitreous fluid and overexpression in the retina of diabetic patients. Arch Ophthalmol 2008. 126(8):1076-1081. [DOD] [CrossRef]
- Sasongko MB, Wong TY, Nguyen TT, Kawasaki R, Jenkins A, Shaw J, Wang JJ. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care 2011. 34(2):474-479. [DOD] [CrossRef]
- Hu A, Luo Y, Li T, Guo X, Ding X, Zhu X, Wang X, Tang S. Low serum apolipoprotein A1/B ratio is associated with proliferative diabetic retinopathy in type 2 diabetes. Graefes Arch Clin Exp Ophthalmol 2012. 250(7):957-962. [DOD] [CrossRef]
- Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol 1999. 19(12):2981-2992. [DOD] [CrossRef]
- Shah PK, Kaul S, Nilsson J, Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part II. Circulation 2001.104(2):2498-2502. [DOD] [CrossRef]
- Hill SA, McQueen MJ. Reverse cholesterol transport - a review of the process and its clinical implications. Clin Biochem 1997. 30(7):517-525. [DOD] [CrossRef]
- Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis 2006. 12:1319-1333. [DOD]
- Robbesyn F, Auge N, Vindis C, Cantero AV, Barbaras R, Negre-Salvayre A, Salvayre R. High-density lipoproteins prevent the oxidized low-density lipoprotein-induced epidermal growth factor receptor activation and subsequent matrix metalloproteinase-2 upregulation. Arterioscler Thromb Vasc Biol 2005. 25(8):1206-1212. [DOD] [CrossRef]
- Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med 2006. 259(5):437-446. [DOD] [CrossRef]
- McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 2008. 372(9634):224-233. [DOD] [CrossRef]
- Davidson MH. Apolipoprotein measurements: is more widespread use clinically indicated? Clin Cardiol 2009. 32(9):482-486. [DOD]
- The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984. 251(3):351-364. [DOD] [CrossRef]
- National Heart, Lung, and Blood Institute Consensus Development Conference: Lowering blood cholesterol to prevent heart disease. JAMA 1985. 253(14):2080-2086. [DOD] [CrossRef]
- Duncan LJ, Cullen JF, Ireland JT, Nolan J, Clarke BF, Oliver MF. A three-year trial of atromid therapy in exudative diabetic retinopathy. Diabetes 1968. 17(7):458-467. [DOD]
- Harrold BP, Marmion VJ, Gough KR. A double-blind controlled trial of clofibrate in the treatment of diabetic retinopathy. Diabetes 1969. 18(5):285-291. [DOD]
- Cullen JF, Town SM, Campbell CJ. Double-blind trial of Atromid-S in exudative diabetic retinopathy. Trans Ophthalmol Soc UK 1994. 94(2):554-562. [DOD]
- Freyberger H, Schifferdecker E, Schatz H. Regression of hard exudates in diabetic background retinopathy in therapy with etofibrate antilipemic agent. Med Klin 1994. 89(11):594-597. [DOD]
- Emmerich KH, Poritis N, Stelmane I, Klindzane M, Erbler H, Goldsteine J, Goertelmeyer R. Efficacy and safety of etofibrate in patients with non-proliferative diabetic retinopathy. Klin Monatblatter Augenheilkunde 2009. 226(7):561-567. [DOD] [CrossRef]
- Havel E, Rencova J, Novak L, Blaha V, Solichova D, Bratova M, Zadak Z. Serum lipoproteins lowering and diabetic exudative retinopathy. Atherosclerosis 1997. 134(1-2):309. [DOD] [CrossRef]
- Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomized controlled trial. Lancet 2007. 370(9600):1687-1697. [DOD] [CrossRef]
- Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004. 110(2):227-239. [DOD] [CrossRef]
- Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyörälä K, Miettinen T, Wilhelmsen L, Olsson AG, et al. Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994. 344(8934):1383-1389. [DOD]
- Gordon B, Chang S, Kavanagh M, Berrocal M, Yannuzzi L, Robertson C, Drexler A. The effect of lipid lowering on diabetic retinopathy. Am J Ophthalmol 1991. 112(4):385-391. [DOD]
- Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract 2002. 56(1):1-11. [DOD] [CrossRef]
- Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol 2004. 137(4):675-682. [DOD]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004. 364(9435):685-696. [DOD] [CrossRef]
- Zhang J, McGwin G. Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol 2007. 125(8):1096-1099. [DOD] [CrossRef]
- ACCORD Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010. 363(3):233-244. [DOD] [CrossRef]
- Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999. 353(9153):617-622. [DOD] [CrossRef]
This article has been cited by other articles:

|
ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases
Gong Y, Fu Z, Liegl R, Chen J, Hellström A, Smith LE
Am J Clin Nutr 2017. In press
|
|

|
Evaluation of kidney function and risk factors of retinopathy in Type 2 diabetes mellitus people in South Africa
Ganjifrockwala FA, Joseph JT, George G
Diabetes Res Clin Pract 2017. 127:218-223
|
|

|
Visual and Morphologic Outcomes in Eyes with Hard Exudate in the Comparison of Age-Related Macular Degeneration Treatments Trials
Daniel E, Grunwald JE, Kim BJ, Maguire MG, Jaffe GJ, Toth CA, Ferris FL
Ophthalmol Retina 2017. 1(1):25-33
|
|

|
A study on relationship between severity of diabetic retinopathy and subclinical hypothyroidism
Borooah M, Phukan S
Int J Res Med Sci 2017. 5(5):1818-1822
|
|

|
Serum total superoxide dismutase enzyme activity in type 2 diabetic patients with retinopathy in Mthatha region of the Eastern Cape Province of South Africa
Ganjifrockwala FA, Joseph JT, George G
Biomed Res 2017. In press
|
|

|
Review: adiponectin in retinopathy
Fu Z, Gong Y, Löfqvist C, Hellström A, Smith LE
Biochim Biophys Acta 2016. 1862(8):1392-1400
|
|

|
Serum Oxidized LDL Levels in Type 2 Diabetic Patients with Retinopathy in Mthatha Region of the Eastern Cape Province of South Africa
Ganjifrockwala F, Joseph J, George G
Oxid Med Cell Longev 2016. 2016:2063103
|
|

|
Correlations between serum vaspin and type 2 diabetic retinopathy
Cheng J, Qi J, Liang J
Biomed Res 2016. In press
|
|

|
Influence of Age at Diagnosis and Time-Dependent Risk Factors on the Development of Diabetic Retinopathy in Patients with Type 1 Diabetes
Forga L, Goni MJ, Ibanez B, Cambra K, Garcia-Mouriz M, Iriarte A
J Diabetes Res 2016. 2016:9898309
|
|

|
Prevalence and risk factors associated with retinopathy in diabetic patients at Parirenyatwa Hospital outpatients' clinic in Harare, Zimbabwe
Machingura PI, Macheka B, Mukona M, Mateveke K, Okwanga PN, Gomo E
Arch Med Biomed Res 2016. 3(2):104-111
|
|
.jpg)
|
Current concepts regarding developmental mechanisms in diabetic retinopathy in Taiwan
Chen SY, Hsu YM, Lin YJ, Huang YC, Chen CJ, Lin WD, Liao WL, Chen YT, Lin WY, Liu YH, Yang JS, Sheu JC, Tsai FJ
Biomedicine (Taipei) 2016. 6(2):7
|
|

|
The glutathione reductase ( GSR ) polymorphisms (rs1002149 and rs8191009) and diabetic retinopathy in Slovenian subjects with type 2 diabetes mellitus
Cilensek I, Ramus SM, Petrovic MG, Petrovic D
Biomed Genet Genom 2016. 1(1):1-5
|
|

|
A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase
Ibrahim AS, Elshafey S, Sellak H, Hussein KA, El-Sherbiny M, Abdelsaid M, Rizk N, Beasley S, Tawfik AM, Smith SB, Al-Shabrawey M
J Lipid Res 2015. 56(3):599-611
|
|

|
Cross Talk between Lipid Metabolism and Inflammatory Markers in Patients with Diabetic Retinopathy
Crosby-Nwaobi R, Chatziralli I, Sergentanis T, Dew T, Forbes A, Sivaprasad S
J Diabetes Res 2015. 2015:191382
|
|

|
Retinopathy is a Strong Determinant of Atherosclerosis in Type 2 Diabetes: Correlation with Carotid Intima Media Thickness
Saif A, Karawya S, Abdelhamid A
Endocr Pract 2015. 21(3):226-230
|
|

|
Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy
Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, Ding Y, Liu Q
Cell Physiol Biochem 2014. 34(5):1733-1740
|
|
|
Prevalence of Dyslipidaemia in Type 2 Diabetes Mellitus Patients and Its Association to Diabetic Retinopathy in a Malaysian Tertiary Hospital
Samsudin IN, Saleh RM, Thambiah SC, Hamzah AS, Khalik WF, George E
Malay J Med Health Sci 2014. 10(2):47-51
|
|

|
Renoprotective effect of atorvastatin on STZ-diabetic rats through attenuating kidney-associated dysmetabolism
Zhou S, Zhao P, Li Y, Deng T, Tian L, Li H
Eur J Pharmacol 2014. 740:9-14
|
|
|