Chapter I. Diabetic Peripheral Neuropathy
| Rev Diabet Stud,
2015,
12(1-2):29-47 |
DOI 10.1900/RDS.2015.12.29 |
Distal Sensorimotor Neuropathy: Improvements in Diagnosis
Prashanth R. J. Vas1, Sanjeev Sharma2, Gerry Rayman2
1Kings College Hospital, London, United Kingdom
2Ipswich Hospital NHS Trust, Ipswich, United Kingdom
Address correspondence to: Prashanth R. J. Vas, Kings College Hospital, London, UK, e-mail: prashanth.vas@nhs.net
Manuscript submitted April 17, 2015; resubmitted April 29, 2015; accepted April 30, 2015.
Keywords: diabetic neuropathy, small fiber neuropathy, axon reflex test, nerve conduction, nerve fiber density, laser Doppler imager flare, corneal confocal microscopy, electromyography, sudomotor function
Abstract
Neurological complications of diabetes are common, affecting up to 50% of people with diabetes. In these patients, diabetic sensorimotor neuropathy (DSPN) is by far the most frequent complication. Detecting DSPN has traditionally been a clinical exercise that is based on signs and symptoms. However, the appearance of morphometric and neurophysiological techniques along with composite scoring systems and new screening tools has induced a paradigm change in the detection and stratification of DSPN and our understanding of its natural history and etiopathogenesis. These newer techniques have provided further evidence that changes in small nerve fiber structure and function precede large fiber changes in diabetes. Although useful, the challenge for the use of these new techniques will be their sensitivity and specificity when widely adopted and ultimately, their ability to demonstrate improvement when pathogenic mechanisms are corrected. Concurrently, we have also witnessed an emergence of simpler screening tools or methods that are mainly aimed at quicker detection of large fiber neuropathy in the outpatient setting. In this review, we have focused on techniques and tools that receive particular attention in the current literature, their use in research and potential use in the clinical environment.
Abbreviations: AAN – American Academy of Neurology; ADA – American Diabetes Association; ADPN – adiponectin; AKR1 B1 – aldo-keto reductase family member B1; AUC - area under the curve; CCM – in vivo corneal confocal microscopy; CHEPS – contact heat-evoked potentials; DFNS – German Research Network on Neuropathic Pain; DN – diabetic neuropathy; DNS – Diabetic Neuropathy Symptom Score; DSPN – diabetic sensorimotor neuropathy; ELMO1 – engulfment and cell motility 1; EMG – electromyography; ESC – electrochemical skin conductance; HRV - heart rate variability; IENFD – intraepidermal nerve fiber density; IpTT – Ipswich Touch Test; LDIflare – laser Doppler imager flare; LFN – large fiber neuropathy; MDNS – Michigan Diabetic Neuropathy Score; MF – 10 gm monofilament; MNCV – motor nerve conduction velocity; MNSI – Michigan Neuropathy Screening Instrument; mTCNS – modified Toronto Clinical Neuropathy Score; NCS – nerve conduction studies; NDS – Neuropathy Deficit Score; NeuPSIG – Neuropathic Pain Special Interest Group of the International Association for the Study of Pain; NICE – National Institute for Health and Care Excel-lence; NIS – Neuropathy Impairment Score; NSS-LL – Neuropathy Symptom Score of Lower Limbs; QLQ-CIPN20 – quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy; QSART – quantitative sudomotor axon reflex test; QST – quantitative sensory tests; RR – interbeat; SCN9A – sodium channel, voltage-gated, type 9 alpha; SFN – small fiber neuropathy; SNAP – sensory nerve action potential; SNCV – sural nerve conduction velocity; SSR – sympathetic skin response; TCNS – Toronto Clinical Neuropathy Score; TRPA1 – transient receptor potential cation channel A1; UENS – Utah early neuropathy score; VGEF – vascular endothelial growth factor
1. Introduction
Diabetic neuropathy (DN) is arguably the most common complication of diabetes; it is also significant because of its associated morbidity and mortality [1, 2]. It is estimated that up to 50% of people with diabetes ultimately develop neuropathy; of these patients 50% are asymptomatic [1, 3]. Whilst acute diabetic neuropathies nearly always present with clear symptoms well recognizable by diabetes specialists, it is the gradually progressive neuropathy with silent onset that predominates, and is often noted only when it is well advanced. Also, acute diabetic neuropathies are associated with considerable morbidity, but gradually progressive neuropathies cause the bulk of the morbidity and mortality. It is now well understood that in the latter case there is a significant discordance between pathological severity and clinical features. However, because of the heterogeneous nature of the various diabetic neuropathies and the myriad of features, disease classification and characterization is difficult [4].
Length-dependent distal sensory neuropathy is the most common form accounting for approximately 80% of DN cases. It is associated with the greatest morbidity, mortality, and costs as it puts patients on a path towards loss of protective sensation, foot deformity, risk of injury, and infection. Ultimately, this path leads to foot ulceration, amputation, and death. The life expectancy of patients with neuropathic foot ulceration is approximately 50% at 5 years. This outcome is worse than many of the major cancers, including breast, colon, and prostate [5]. Eventually, distal sensory diabetic neuropathy can result in Charcot neuroarthropathy, a disabling and depressing chronic complication [3].
In recent years, the development of modern investigation methods for nerve fiber structure and function has revealed pathological changes occurring prior to the development of symptoms or signs of neuropathy, in particular those in small nerve fibers [6]. These findings are challenging previously established classification systems and diagnostic algorithms. In this paper, we discuss the recent improvements in the field of diabetic neuropathy, with a specific focus on distal sensorimotor neuropathy (DSPN).
2. Definition and severity assessment of diabetic sensorimotor neuropathy (DSPN)
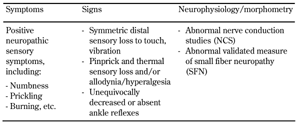
According to the classic concept by P. K. Thomas, diabetic neuropathy is a symmetric distal polyneuropathy with predominant sensory and relatively minor motor nerve involvement [7]. A statement by the American Diabetes Association in 2005 defined diabetic polyneuropathy as a clinical diagnosis based on the presence of symptoms and/or signs of peripheral neural dysfunction in people with diabetes after the exclusion of other causes (Table 1) [3]. In this classification, generalized symmetric polyneuropathy of diabetes was divided into three variants:
1. Chronic sensorimotor polyneuropathy
2. Acute sensory neuropathy
3. Autonomic neuropathy
However, these concepts do not include specific diagnostic criteria to confirm or exclude the diagnosis, nor do they provide criteria to determine severity. In 2005, The American Academy of Neurology developed a case definition and investigation protocol for distal symmetrical polyneuropathy, but the primary aim was to ensure future research studies to approach the question with greater consistency of case selection [8]. The authors concluded that the best approach to define DSPN would be an ordered set of definitions that include key features for the presence of neuropathic symptoms, ankle reflexes, distal sensation, muscle weakness/atrophy, and nerve conduction findings, and that are ranked by the likelihood of disease appearance [8]. According to this concept, an ordinal scale was developed that included 4 stages of DSPN probability, from highest ("++++") to lowest ("+"), with a recommendation to limit the enrolment of subjects into clinical research studies to those at the highest ordinal probability [8].
Table
1.
Symptoms, signs, and morphometry of distal sensimotor polyneuropathy (DSPN) |
|
 |
 |
2.1 Toronto consensus on the determination of diabetic neuropathy
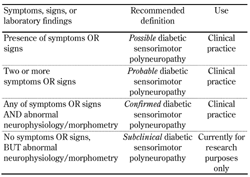
The Toronto expert panel convened in 2009 to update and provide clear definitions and case characterizations of diabetic neuropathy [9]. They proposed separate definitions for typical diabetic polyneuropathy (i.e. the classic DSPN) and for atypical neuropathies. DSPN was defined as a symmetrical, length-dependent sensorimotor polyneuropathy attributable to metabolic and microvascular alterations resulting from chronic glycemic exposure and cardiovascular risk covariates. It was recommended that DSPN is classified into i) possible, ii) probable, and iii) confirmed DSPN, and they added a fourth category, iv) subclinical DSPN (Table 2) [9].
Table
2.
Diabetes-typical distal sensorimotor polyneuropathy (DSPN) according to the Toronto consensus definition [9] |
|
 |
 |
2.2 Severity assessment
If DSPN is confirmed once, its severity needs to be determined. Until the Toronto consensus, there were no agreed guidelines on the use of validated or objective tools to ascertain the severity of DSPN. The panel recommended the degree of nerve conduction abnormality as the minimal standard, but also supported an alternative approach suggested by Dyck in 1988. The latter approach grades the severity of DSPN from 0 to 2b, but does not take into account small fiber measures [9].
Assessment of severity using composite clinical scoring systems. The use of neuropathy composite scoring systems is one way of objectively measuring DSPN severity. Many of these systems have been developed and validated, but the following have found widespread application in both epidemiologic studies and clinical research:
- Neuropathy Deficit Score (NDS) of Boulton
- Michigan Neuropathy Screening Instrument (MNSI)
- Toronto Clinical neuropathy Score (TCNS)
- Diabetic Neuropathy Symptom Score (DNS)
- Neuropathy Impairment Score (NIS)
- Neuropathy Symptom Score of lower limbs (NSS-LL)
- Utah Early Neuropathy Score (UENS)
These systems are simple to administer, have very good concordance between operators when stratifying patients into different neuropathy severity levels, and may help provide an independent reference. Some of the newer scores have a reported ability to detect temporal change. The use of composite neuropathy scores is recommended in the Toronto consensus for measurement of DSPN severity [9].
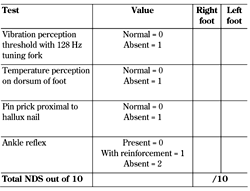
Neuropathy Deficit (or Disability) Score (NDS). The NDS is a simple tool to grade the severity of neuropathy based on objective clinical examination assessing qualitative vibration perception, temperature differentiation, pinprick sensation, and presence of ankle reflexes [10-11]. No points are awarded for preserved sensation, but if impaired or absent 1 point is allocated per foot, except for ankle reflexes where 2 points are awarded if absent and 1 point if reflexes are present after reinforcement, thus giving a total of 10 points (Table 3). The following score system has been established:
0-2: clinical neuropathy is excluded
3-5: mild neuropathy
6-8: moderate neuropathy
>8: severe neuropathy
A score of >6 is also said to correlate well with a vibration perception threshold of >25 volts [11]. Many recent studies validating small fiber measures have used the NDS to denote the presence and stratify the severity of clinical neuropathy [12-13]. However, it must be noted that the NDS is different from the Neuropathy Disability Score developed by the Mayo Clinic, another validated method for DSPN assessment, with a reported sensitivity of 48% and a specificity of 91% [14-15].
Table
3.
Neuropathy Deficit (Disability) Score (NDS) [11] |
|
 |
 |
Michigan neuropathy Screening Instrument (MNSI) and Michigan Diabetic Neuropathy Score (MDNS). MNSI consists of two separate assessments, a 15 point history questionnaire which is self-administered by the patient and physical assessments parameters of foot inspection, vibration perception assessment using a 128 Hz tuning fork, and monofilament testing [16]. Out of possible score of 8, scores >2.5 are considered to suggest DSPN [16]. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study used a standard of neurological examination evaluated against abnormal nerve conduction parameters. A combined MSNI value of >2.8 produced an area under the curve (AUC) of 0.81, with sensitivity of 43%, specificity 95%, positive predictive value 80%, and negative predictive value 80%, and explained a variance of r2 = 27% [17]. It must be noted that the MNSI was developed primarily as a screening instrument. The Michigan Diabetic Neuropathy Score (MDNS) may be further used in those patients who have been screened positive with the MNSI for confirming DSPN; it has a neurological quantitative assessment component coupled with nerve conduction studies.
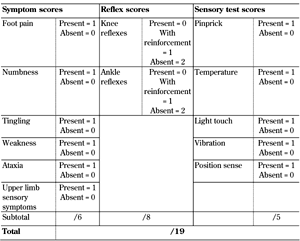
Toronto Clinical Neuropathy Score (TCNS). The TCNS was initially developed as part of a screening tool for diabetic neuropathy. It consists of three parameters:
1. Symptom scores (present = 1, absent = 0)
2. Reflex scores (present = 2, reduced = 1, absent = 0)
3. Sensory test scores (present = 1, absent = 0)
The possible maximum score is 19 (Table 4) [18]. Importantly, it remains one of the few scores that have been validated against morphometry, with a significant negative correlation with sural nerve fiber density (r2 = -0.256, p < 0.0001). Subsequently, the authors published a modification, the mTCNS, to better capture the early sensory abnormalities of DSPN, and to eliminate muscle reflex tests which are notoriously variable between raters [19]. In a recent study comparing seven neuropathy composite scores in individuals with impaired glucose tolerance and symptomatic early neuropathy of less than 2 years' duration, mTCNS was the strongest discriminant, with an AUC of 0.99 (p = 0.006) for all subjects with neuropathy [20]. A cut off value of 3 had a sensitivity of 98% and specificity of 97%, with a positive predictive value of 99% and negative predictive value of 94% [20].
Table
4.
Toronto Clinical Neuropathy Score by Bril and Perkins [18] |
|
 |
 |
Neuropathy Impairment Score of the Lower Limb (NIS-LL) and NIS (LL)+7. The NIS-LL is a subset of the NIS; it allows objective assessment of the lower limbs, the region most commonly affected, and removal of variables not specific for DSPN. The NIS itself was an adaptation of the earlier Neuropathy Disability Score developed by Dyck and colleagues from the Mayo Clinic group by replacing those tests that are normal in DSPN [21-22]. The NIS-LL is complex to administer and quantifies by attributing a score ranging from ‘0’ (no DSPN) to 88 (complete impairment). The components of the NIS-LL are sensation (vibration, pinprick, touch, pressure, joint position), muscle tendon reflexes (knee and ankle), and muscle group power assessments (hip flexion, hip extension, knee flexion, knee extension, ankle dorsiflexors, ankle planter flexors, toe extensors, toe flexors). In the Rochester Diabetic Neuropathy Study, Dyck and colleagues adapted the NIS-LL, adding in vibration detection thresholds, specific parameters from nerve conduction studies (NCS), and heart rate variability with breathing to yield the NIS(LL)+7 [4, 23]. A further advantage of the NIS(LL)+7 is its ability to assess dynamic and temporal changes in DSPN severity. For the Rochester study cohort, the reported overall 2-year mean and standard deviation change in NIS(LL)+7 were 1.08 points and 3.57 points, respectively [23]. The authors also reported that the diabetes individuals worsened their NIS(LL)+7 by 0.34 points/year, while those with DSPN had their scores reduce by 0.85 points/year [23].
Utah Early Neuropathy Score (UENS). Most neuropathy composite scores assess reflexes, motor strength as well as sensory symptoms and signs. Therefore, they have a significant large fiber bias. This may result in a diminished sensitivity to early neuropathy which is predominantly related to sensory perception. The Utah Early Neuropathy Scale (UENS) was developed by a North American collaborative with the specific aim of detecting and quantifying early small fiber-mediated sensory neuropathy and to recognize modest changes in sensory severity [24]. Unusually, for a composite score, it was designed at the outset to allow detection of temporal changes in anatomical distribution of pin prick sensation [24]. Consequently, it puts more importance on cutaneous pin prick sensation and allodynia, conferring it 26 out of possible 42 points, and allocating only 16 points to large fiber modalities and motor examination. Accordingly, validation data demonstrated good correlation with sural sensory amplitude (r = 0.40, p < 0.002) and with intraepidermal nerve fiber density (r = 0.43, p < 0.001) [24]. However, it also correlated strongly with the MDNS (r = 0.89, p < 0.0001) and the NIS-LL (r = 0.86, p < 0.0001). When the criterion for DSPN was defined as symptoms of neuropathy, with confirmed abnormalities by two or more electrodiagnostic, electrophysiological, or histological tests, the UENS had a sensitivity of 92% (higher than 67% for MDNS and 81% for NIS-LL). It also had a specificity similar to other scores (unreported in the paper, but estimated around 75% from the ROC) and an AUC of 0.88 (MDNS 0.77 and NIS-LL 0.81) [24].
3. Diagnostic techniques for DSPN
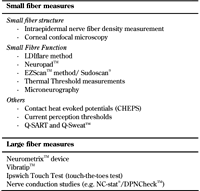
As described previously, there is now increasing evidence to suggest that neuropathy of the smaller unmyelinated Aδ and C fibers may precede large fiber neuropathy, especially in type 2 diabetes and in states of impaired glucose tolerance [6, 25-28]. These fibers comprise 75-90% of the peripheral nerves, and mediate pain and temperature as well as autonomic function [27, 29]. Researchers are increasingly convinced that small fiber neuropathy (SFN) may be the ‘microalbuminuric’ equivalent of DSPN, and may have a potential future role in studies of putative agents aimed at treating diabetic neuropathy. This has led to an increased effort in developing techniques for accurate characterization of SFN since traditionally used clinical laboratory measures (such as NCS) do not identify the disease early enough, and the previously discussed composite scores are not specific for SFN. For the purpose of this review, we have divided the methods for investigating small fibers into those measuring small fiber structure and those measuring function (Table 5). Although current equipment gives objective, reproducible, and quantitative measures of large fiber function, NCS is only accessible in neurophysiology laboratories. Therefore, in practice it is only used when clinical presentation is atypical or in the context of a research study. Recently, portable devices such as the DPN-StatTM device have added in a dimension of bedside accessibility to NCS; their utility is briefly discussed in section 3.3.
Table
5.
New techniques in distal sensorimotor polyneuropathy assessment |
|
 |
 |
3.1 Small fiber neuropathy (SFN) - definition and classification
There is no agreed definition of small fiber neuropathy [30-31]. In the literature, definitions have reflected the various methods used by research teams, including physiological methods to asses small nerve fiber function (or dysfunction) and quantitative (but psychophysical) measures of thermal and pain thresholds and abnormalities on skin biopsy quantification [31]. Therefore, the presence of SFN is determined by centile cut-off values appropriate for age and gender, rather than by a clear clinical definition [30, 32-33].
The clinical hallmark of lower limb small fiber neuropathy is a disturbance in pain sensation. However, numerous studies have shown that disordered small fiber function and/or structure occurs early in the course of diabetes, even with impaired glucose tolerance, and can even be present in the absence of disordered pain sensation [34-35]. In patients with disordered pain sensation characteristic of SFN, nerve conduction tests are often normal, and have a supportive role to exclude differential etiologies such as chronic inflammatory demyelinating polyneuropathy [21, 31].
To date, there is no standardized classification of SFN, although the Toronto consensus includes abnormality in a validated measure of small fiber neuropathy in its classification of DSPN (Tables 1 and 2). Upcoming studies are demonstrating progression of SFN to higher degrees of neuropathy in diabetes, but evidence from more longitudinal studies is required to draw definite conclusions [35-36].
3.2 Methods for assessment of small fiber neuropathy
Intraepidermal nerve fiber density measurement (IENFD). Skin biopsy with measurement of intraepidermal nerve fiber density (IENFD) is now a widely accepted technique to detect SFN [30, 33]. The technique was initially described by McCarthy et al. in 1995, and applied to evaluate DSPN in non-diabetic sensory neuropathies [37]. Since then it has been shown to be an objective and reliable marker of SFN; indeed it is considered the gold standard in some guidelines [38]. The method requires a specimen that is obtained by a 3 mm punch biopsy of hairy skin at the distal leg usually 10 cm above the lateral malleolus in the region of the sural nerve. The specimen is immunostained for protein gene peptide 9.5 (PGP 9.5), a panaxonal neuronal antigen [39]. The bright field immunochemistry protocol by Lauria et al. is the most widely used. Quantification of linear IENFD results are expressed in number of fibers per millimeter in at least three sections of 50 μm [33, 39-40]. Another technique, indirect immunofluorescence with optical fluorescence or confocal microscopy, is also used [40]. The procedure is simple to perform and well-tolerated; healing occurs within 7-10 days. [29, 38]. The complication rate is low; the most common reported side effect is mild wound infection recoverable with topical antibiotic therapy [38]. Kennedy et al. reported a relationship between IENFD and DSPN severity in diabetic candidates awaiting pancreas transplantation compared with control subjects [41].
Operating characteristics of IENFD in detecting neuropathy are robust, sensitivity is between 60% and 95% and specificity between 90% and 95% [30-31, 42]. In the Lifestyle Intervention for Pre-Diabetic Neuropathy study, there was up to 1.4 ± 2.3 fibers/mm (p < 0.004) improvement in IENFD after 1 year of treatment, indicating that this technique could detect and quantify cutaneous reinnervation [43]. The same group have recently demonstrated that in established diabetes a structured, supervised weekly exercise regime could lead to improvement in IENFD (1.5 ± 3.6 fibers/mm diabetic subjects vs. -0.1 ± 3.2 fibers/mm controls, p = 0.03) [44]. This improvement has also been shown in patients with metabolic syndrome [45], confirming that IENFD is an useful marker with potential to detect SFN reversal. However, these studies were uncontrolled. Hence, controlled trials are necessary to demonstrate efficacy and true effect.
An additional advantage is the availability of robust worldwide normative data and a standardized laboratory protocol [39]. The main drawback is its invasive nature and the requirement for a specialist laboratory background, as reported by many. Nevertheless, its demonstration of reversibility of small nerve fiber dysfunction nominates this technique as standard for investigation of new therapies to prevent or reverse early DSN.
In vivo corneal confocal microscopy (CCM). This has been shown to be a rapid, non-invasive ophthalmic technique that can accurately quantify corneal innervation in the human subbasal plexus [46]. In diabetes, it has been shown that reductions in corneal innervation occur early in the disease, are symmetrical between left and right eye, worsen with increasing severity of DSPN, and that such changes are parallel to similar changes in IEFND in the feet [47-49]. Tavakoli et al. from the Manchester group have demonstrated that all parameters measured in CCM correlate strongly with NDS and indeed with DPSN severity (corneal nerve fiber density r = -0.475, p < 0.0001; nerve branch density r = -0.511, p < 0.0001; nerve fiber length r = -0.581, p < 0.0001) [48]. Receiver operating characteristic curve analysis for the diagnosis of neuropathy (using NDS >3 to indicate clinical neuropathy) defined a sensitivity of 82% and specificity of 52%. If patients with foot ulcer risk were defined (NDS >6 as the standard), sensitivity increased to 71% and specificity to 64% [48].
Another recent study reported that corneal nerve fiber length was the best discriminator of the CCM variables, with an optimized sensitivity of 85% and specificity of 84% for identifying DSPN by using CCM [50]. They also reported on the advantage of separate thresholds to respectively identify or exclude DSPN. Whilst a single threshold offered clinically acceptable operating characteristics, separate thresholds may have more robust performance with sufficient predictive validity to identify individuals who are at risk of developing DSPN [50]. Using CCM variables, studies have documented improvement in terms of nerve fiber repair with tight glycemic control [51], 6 months after pancreas transplantation [52], after simultaneous kidney-pancreas transplantation [53], and with insulin pump therapy [54]. The manual counting method is time-consuming and costly, but newer automated methods have been validated successfully, with reported area under the curve for identifying DSPN of 0.82 for the manual method and 0.80 for the automated algorithm [55-57].
In a cohort of recently diagnosed subjects with type 2 diabetes (with a mean duration of diabetes of 2.1 ± 1.8 years), Ziegler et al. showed that CCM and IENFD were reduced below the 2.5th percentile in 21% and 14% of patients, respectively [58]. Surprisingly, there was poor concordance between those abnormal for CCM and IENFD, confirming that small nerve fiber structural change is patchy and heterogeneous in nature [58]. With more widespread research application of CCM, newer image acquisition algorithms are being generated; some encompassing larger scanning areas than the traditional image frames of 0.15 m2 [28, 58-59].
Laser Doppler imager flare (LDIflare). Based on the observation that the neurogenic axon reflex-mediated flare response is abnormal in individuals with SFN, the size of the stimulated axon reflex flare has been proposed as a non-invasive measure of small fiber neuropathy [60]. When action potentials are generated in nerve endings of C-fibers or the Aδ fibers, they are conducted orthodromically and transmitted antidromically, exciting the adjacent neurons [61]. This results in the release of vasoactive neuropeptides such as calcitonin gene-related peptide, substance P, and histamine, provoking a vasodilatory flare response [61-62]. This response can be quantitated by determining the induced flare area and intensity using laser Doppler imaging.
In laboratory studies, using iontophoresis techniques, it has been shown that neurovascular vasodilation accounts for up to 30% of the total response to acetylcholine, and it is significantly reduced in DSPN [63]. The LDIflare technique is the clinical application of this principle to measure the induced flare area at the dorsal foot skin. It utilizes a scanning laser device and skin heating as the nociceptive stimulus [64]. As with IENFD and CCM, LDIflare has been validated against large and small fiber markers and bears a strong correlation to IENFD (r = 0.77, p < 0.001) [62]. It can detect abnormal small fiber function in increasing severity of diabetic neuropathy [64-65], impaired glucose tolerance with normal thermal thresholds [26], and as reported recently, in non-diabetic non-neuropathic individuals with hypertriglyceridemia [66]. Studies have shown that glycemic control and HbA1c have a strong relationship with LDIflare results [67-68].
In the detection of clinical neuropathy, the LDIflare technique has a sensitivity of 70-75%, specificity of 66-85% [32, 68], positive predictive value of 74%, and negative predictive value of 86% [32], depending on the methodology used. The modified LDIflare technique employs a higher skin heating temperature, but for a shorter duration (47oC for 3 minutes V 44oC for 20 minutes), and can therefore be administered easily in a clinic setting [69]. It may also have a role in diagnosis and quantification of chemotherapy-induced peripheral neuropathy. Also, it correlates with the QLQ-CIPN20 symptom scores in those patients receiving platinum-based therapies (r = 0.81, p = 0.001) and taxane-based agents (r = 0.58, p = 0.027), when sural nerve conduction velocity and amplitude do not [70].
Sudomotor function assessments. Up to 56% of type 1 diabetes patients suffer from a reduction in active foot skin sweat glands, and up to 40% have a reduced sweat evaporation rate [71]. Abnormalities of C-fibers in DSPN leads to sudomotor dysfunction manifesting as a reduction in plantar sweating, plantar anhidrosis, and dry skin [72]. Sudomotor function tests provide information on peripheral autonomic function. In specialist centers, they are currently used as adjuvant or screening tests for DSPN [73]. The quantitative sudomotor axon reflex test (QSART) [74-75] and the commercially available Q-Sweat [76] have been in clinical application for more than a decade. However, these procedures require expensive equipment and need purpose-built lab space.
Detection of sympathetic skin response (SSR) in the eccrine sweat gland is another useful method, but also requires dedicated testing laboratories [77-78]. A study comparing QSART and SSR found similar rates of detection of approximately 50% in a group with DSPN defined by the presence of symmetrical distal sensory disturbances and absent Achilles tendon reflexes [75]. More recently, the development of simple techniques that can be reliably administered in a clinical setting has leveraged the methods in clinical application. In this review, we have focused on the recently introduced Neuropad® and Sudoscan® methodologies.
Neuropad®. Neuropad® (Trigocare International GmbH, Germany) is a simple bedside screening test for DSPN, providing a qualitative/categorical indication of sudomotor dysfunction. A plaster is adhered to the plantar surface of the forefoot for 10 minutes; it changes color from blue to pink as the impregnated anhydrous cobalt II compound comes in contact with foot skin sweat [79]. Response is determined as normal (no neuropathy) if the color completely changes to pink or abnormal (presence of neuropathy) for absent or incomplete patchy color change [79]. The Neuropad® abnormal patchy/absent response has been shown to have a 70-95%% sensitivity, 50-71% specificity [80-83], and 98% negative predictive value for both large and small fiber DSPN (using clinical examination as reference standard). For small fiber dysfunction, the values are 86%, 71%, and 93%, respectively [82].
In an effort to develop a more precise quantitative analysis of color change, researchers from Manchester University have developed the sudometrics image analysis algorithm that uses digital analysis of a Neuropad® photograph. It can quantify the Neuropad® response in a range from 0% to 100% instead of the established categorical value [84]. In a recent paper, the authors have reported that this method improved overall diagnostic efficacy of the Neuropad® in detecting DSPN, especially small fiber neuropathy and autonomic neuropathy [84]. In comparison to CCM parameters, which served as reference standard, sensitivity and specificity of the Neuropad® was 88% and 78%, respectively; it was 88% and 83%, respectively, when SNAP was used as standard [84]. Importantly, the visual nature of the Neuropad® may have an additional role in patient self-examination and education about DSPN [85-87].
Sudoscan™. Sudoscan™ (Impeto Medical, France) is an FDA approved device for the assessment of sudomotor function as a marker of DSPN severity. The principle is based on measuring the electrochemical skin conductance (ESC) between the chloride ions in the sweat of hands and feet which are placed on stainless steel-based plate electrodes of the machine. Results are expressed in µ-Siemens units [88-89]. A low-voltage current (<4 V) is applied through the electrodes, attracting chloride ions from the sweat glands by reverse iontophoresis [89]. An earlier device utilizing the same principle, EZScan™, has shown promise in non-invasive screening for type 2 diabetes and impaired glucose tolerance [90-91]. Studies with Sudoscan have shown that diabetes patients with DSPN have significantly lower ESCs of feet and hands than those without clinical DSPN or healthy controls (56.3 ± 3 vs. 75.9 ± 5.5 and 84.4 ± 0.9, p < 0.0001 for feet and 51.9 ± 2.4 vs. 67.5 ± 4.3 and 73.1 ± 0.8, p < 0.0001 for hands) [89]. Furthermore, increasing NIS-LL scores were associated with decreasing ESC values [89]. It has a reported sensitivity of 77-78% and specificity of 67-92% for the detection of clinical DSPN [89, 92]. Using the UENS as reference standard, Sudoscan has similar operating characteristics as IENFD (AUC of 0.76 v 0.75 for IENFD, p = NS) [92]. Furthermore, in the cross-sectional cohort of healthy controls and diabetes individuals, Sudoscan™ demonstrated a moderate but significant correlation with sural serve amplitude (r = 0.34, p < 0.02) [92].
Quantitative sensory tests (QST) for thermal, pain, and vibration perception. These tests are designed to provide a quantitative measure of sensation. They have been shown to provide valuable information for assessing DSPN. Both the San Antonio consensus [93] and the Toronto consensus recommended the use of quantitative sensory tests with thermal thresholds for DSPN diagnosis [9]. Various techniques and devices are available, ranging from the handheld Tiptherm device [94] across current perception threshold devices to the sophisticated computerized instruments such as CASE IV from WR Medical® and the NeuroSensory Analyzer (TSA) from Medoc® [23, 95]. The latter two have the advantage of a large database of accurate normative values.
A drawback of QST is the psychophysical subjective nature, requiring cooperation from the patient. This has resulted in a wide variation of published reproducibility values [30, 96]. Abnormal results suggest a dysfunction somewhere along the sensory pathway, not necessarily directly at the site of testing [96]. However, with good training and standardization of testing methodology, the German Research Network on Neuropathic Pain (DFNS) has shown good test/retest results, enabling inter-observer reliability [97]. Furthermore, QST may have an important role in the quantification of positive sensory symptoms such as allodynia and hyperalgesia [98]. In their recent consensus document, the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain (NeuPSIG) recommended the use of QST for screening for small and large fiber neuropathies, monitoring of somatosensory deficits, and monitoring of evoked pains, allodynia, and hyperalgesia [99]. The group also suggested using QST as the sole test for diagnosis of neuropathic pain. They further highlighted the importance of standardized stimuli and instructions, validated testing algorithms, and reference values [99]. In the 2005 AAN document, the consensus committee noticed too much inconsistency among the studies describing the accuracy of QST, and did not include the methodology in the final case definition of DSPN [8].
Simple handheld vibration perception threshold measurement devices (e.g. neurothesiometer) correlate well with NCS parameters, are quick, reproducible, and painless [100]. Indeed, in some studies, they have shown superior performance to CASE IV (sensitivity for DSPN 70% vs. 49% for CASE IV) [101], and have been validated to predict risk of foot ulceration [102]. Current perception thresholds (Neurometer®) and contact heat-evoked potentials (CHEPS) are new emerging tools with data on reproducibility and its relationship to more established reference parameters [103-104]. The recently developed normative values for CHEPS may allow for wide adoption in research studies [105].
Microneurography. Microneurography is a minimally invasive technique, which allows single-fiber recordings from peripheral axons in conscious subjects [106]. The slow recovery of sodium channels during the relative refractory period shows up as a period of pronounced slowing of conduction velocity, a phase that is more pronounced in C-fibers [107]. Microneurography of C-fibers utilizes this period to judge responsiveness of electrically driven C-units to additional natural stimulation of their receptive fields [107]. Pain from stimulation of cutaneous nociceptive C-fibers and Aδ fibers is felt as superficial pricking or burning in the skin, and is projected with an accuracy of 1-2 cm relative to the receptive fields of the stimulated fiber [107-108]. The technique also allows for functional classification of the C-fibers into mechano-sensitive, mechano-insensitive nociceptors, or sympathetic fibers [106, 109].
There is emergent data on differences in C-fiber subtype ratios between the young and aged human, but broad age- and gender-specific normative values are lacking [106]. Furthermore, validation of the technique with other more accepted markers of DSPN is limited. The finding that specific small nerve fibers play a role in positive symptom generation is exciting. Therefore, microneurography is likely to have a future role as an objective measure of pain and an endpoint in pain pharmacotherapy research. However, microneurography can be time-consuming and difficult, requiring a patient subject and an expert investigator.
Tests for visceral autonomic neuropathy. Our review has concentrated on somatic DSPN, but the importance of diabetic autonomic neuropathy cannot be further emphasized as they are essentially small nerve fibers. Prevalence data vary according to the criterion used, but have been reported to be between 2% and 65% in the cohorts studied, increasing with age and diabetes duration [110]. Evidence of the concomitant presence of cutaneous, cardiac, and visceral autonomic neuropathy in DSPN has been reported [111-112]. Importantly, there is a strong association between cardiac autonomic neuropathy and cardiovascular mortality [2]. Heart rate variability (HRV) employing interbeat (RR) intervals of the electrocardiogram is by far the most used technique with a reported specificity of 80% [9]. In the quest for variability, this method can be performed by applying the deep breathing technique, valsalva manoeuver, or lying-standing [110]. HRV is an early symptom of cardiac autonomic neuropathy [113]. Orthostatic hypotension is another easily measurable parameter that is defined as a fall in blood pressure >30 mm Hg systolic or >10 mm Hg diastolic blood pressure in response to a postural change, usually from supine to standing; it is another recommended method in the Toronto Consensus [110]. Other tests recommended in the Toronto consensus include measurement of baroreceptor sensitivity, muscle sympathetic nerve activity assessment, measurement of plasma levels of catecholamines, and cardiac sympathetic scintigraphic mapping [9]. There is increasing recognition that visceral autonomic neuropathy may be less frequent than skin/sudomotor autonomic functions or lag behind in DSPN, while skin/sudomotor autonomic functions are frequently impaired [111, 114].
3.3 Methods for assessment of large fiber neuropathy
Routine use of 10 gm monofilament (MF) or a 128 Hz tuning fork is advocated in busy diabetic clinics to confirm the presence of DSPN [115]. However, these devices only detect advanced DSPN. The MF was developed more as a marker for loss of protective sensation, and is a good predictive tool for risk of foot ulceration [116]. In this review, we focus on new bedside tests for large fiber neuropathy, while providing a brief overview of electrodiagnostic improvements.
Electrophysiological studies. Large nerve fibers (A-alpha, A-beta, and A-gamma) mediate touch, vibration, and proprioception, and also innervate muscle spindles; established abnormalities may be detected by clinical examination. For more precise characterization, electrodiagnostic studies of nerve conduction parameters remain the benchmark for the diagnosis of DSPN (and atypical neuropathy). Some researchers consider them an extension of clinical neurological examination [21]. Nerve conduction studies are sensitive, specific, reproducible, and validated measures of DSPN, with the ability to differentiate established distal, axonal, and sensory changes of DSPN from proximal motor demyelination or demyelination causes [117].
Initial studies looking into nerve conduction and nerve morphometry suggested that segmental demyelination along with axon loss was the hallmark of diabetic neuropathy [118-119]. However, Dyck et al. latterly concluded from their studies that segmental demyelination was secondary to axonal degeneration [120-121]. The most distal sensory nerves (sural, plantar) typically provide the first electrodiagnostic evidence of DSPN [21]. Subsequently, progressive changes may develop in the distal sensory and motor nerves and also in upper limb nerves. In the well characterized population of the Rochester Diabetic Neuropathy Study, the most frequent abnormal attributes under the 2.5th and over 97.5th percentile were fibular (peroneal) motor nerve conduction velocity (26.3%), sural sensory nerve action potential (25.4%), tibial MNCV (24.8%), ulnar MNCV (21.3%), fibular F-wave latency (16.9%), and ulnar F latency (16.0%) [122].
The AAN consensus from 2005 suggested a simplified NCS protocol for detection of DSPN. Sural sensory and peroneal motor NCS acquisitions were considered most sensitive, and were recommended as the first line tests. If they remained normal, no further electrodiagnostic studies were recommended [8]. If abnormalities were present, the consensus was to include ulnar and median sensory NCS along with median motor values, and to assess the contralateral limb parameters [8]. Furthermore, electrodiagnostic studies, especially motor conduction velocity, continue to be FDA recommended surrogate endpoints in epidemiologic and neuropathic drug development trials [123].
Muscle pains are common in neuropathy, and 'cramps' have been reported in up to 33% of such individuals [124]. These symptoms are in accordance with peripheral nerve hyper-excitability. Although not routinely used in neurophysiology laboratories, assessment of peripheral nerve hyper-excitability using slow repetitive nerve stimulation (to assess cramp after discharges) is a neurophysiological technique with reported sensitivity of 79% and specificity of 88% [125-126]. F-wave latency may serve as a sensitive indicator of DSPN [127-128]. However, its role in diagnosis and characterization of DSPN remains unclear, and both the AAN consensus and Toronto consensus do not provide specific recommendations on its use.
Features suggestive of demyelination, including significant reduction in motor conduction velocity and prolonged distal motor latency, may be associated with DSPN in some patients. This makes it difficult to differentiate DSPN from the immunologically mediated chronic inflammatory demyelinating polyneuropathy [129]. Electromyography (EMG), the needle electrode examination of muscles, supplements the NCS, but has a limited role in DSPN. Typically, an EMG would be performed to investigate possible other diagnoses in addition to DSPN such as radiculopathy, inflammatory myopathy, or atypical motor neuropathy.
Limitations of electrodiagnostic studies include the need for referral to a neurophysiology lab, the 30-40 minutes testing time, and a degree of patient discomfort. More recently, the NPhys Trial 3 demonstrated that NCS attributes were without significant intra-observer differences, but there were significant inter-observer differences, sometimes within the same neurology department [130]. Robust normative values will help to characterize the presence of DSPN with greater accuracy. Studies have shown a significant effect of age, gender, type of diabetes, and anthropometric measures on NCS [131-132]. Inter-observer variation in NCS acquisition may be reduced with the use of detailed standard reference values and a clearly pre-defined percentile level of abnormality [130]. With recent studies, demonstrating SFN to precede NCS changes and small fibers to possess the ability to regenerate (albeit for temporary periods), the use of NCS measures as preferred endpoints by regulatory authorities is being increasingly debated.
NC-stat®/DPNCheck™. The NC-stat®/DPN Check™ system (Neurometrix, Waltham, MA) is a simple, low-cost, handheld point-of-care device that measures NCS quickly and accurately with basic training, but without the need for laboratory-based, expensive electrodiagnostic equipment. The system consists of a single-use flexible biosensor panel, a handheld device with LED display, software that can download data from the device, and a cable to connect with a computer. It provides information on two NCS measures: 1) sural nerve conduction velocity (SNCV) and 2) sensory nerve action potential (SNAP) amplitude. Previous studies have shown that it has excellent correlations with conventional NCS (r = 0.76 to 0.91, p < 0.001) and excellent intra-rater reliability inter-class coefficients of 0.97 and 0.94 for SNAP and SNCV, respectively [133-135]. It has recently been validated in a study comparing its performance in detecting DSPN with that of the LDIflare technique, the latter measuring small fiber function. The NC-stat®/DPNCheck™ system demonstrated an excellent performance (AUC 0.74 to detect DSPN with NDS > 3) [70]. Although the device has a few limitations (sensory amplitude below 1.5 µV being assigned zero and orthrodromic stimulation of sural nerve), it may have a future role in large cross-sectional studies of newly diagnosed diabetes cohorts, and possibly even regular DSPN screening.
VibraTip™. The VibraTip™ is a small key fob-shaped device intended for DSPN screening during routine annual diabetes checks [136]. Whist in principle it is an electronic proxy of a tuning fork, it differs in that it provides near-silent vibration of constant amplitude [136]. The VibraTip™ device is applied to the tip of the halluces, and provides categorical (qualitative) data on vibration perception. In a cross-sectional diagnostic accuracy study, with quantitative vibration perception threshold and NDS as the gold standards, VibraTip™ had good agreement with the reference tests [136-137]. Relative to the Neurothesiometer, VibraTip™ has a reported sensitivity of 79-100% and specificity of 83-97% [137-139]. It has not yet been fully evaluated against NCS or SFN measures. The VibraTip™ was recently evaluated by the National Institute for Health and Care Excellence (NICE) for the purpose of clinical use in the detection of DSPN. The final recommendation was that, whilst the technology demonstrated promise, submitted and published evidence was insufficient to determine diagnostic superiority or equivalence of VibraTip™ confidently compared with the MF or the 128 Hz tuning fork [136]. Additionally, the committee opined, the device is unlikely to reduce foot examination costs [136].
Ipswich Touch Test. The Ipswich Touch test (IpTT) was developed out of a need for a 'ready-at-hand' neuropathy screening tool for the detection of individuals with the highest risk of foot ulceration, with the specific aim of reducing hospital-acquired foot ulceration [140]. Relative to the vibration perception threshold (VPT), it has good sensitivity (77%) and specificity (90%), which is not significantly different from the established 10 gm monofilament technique (sensitivity (85%) and specificity (88%) when compared with the VPT). Also, it is straightforward enough to be used by relatives, healthcare staff, and friends, thus supporting self-examination and providing patient education [141]. Bowling et al. have also independently validated the IpTT, demonstrating that it produces identical results to Vibratip™ (kappa, κ= 1.0), with almost perfect agreement when compared with the VPT (κ = 0.97, p < 0.001) and the Neuropathy Disability Score (κ = 0.92, p < 0.001) [137]. More recently, a group from Saudi Arabia has evaluated the IpTT as a reliable screening tool for the loss of protective sensation with the ability to overcome barriers in foot risk screening [142]. The IpTT has been recommended in the recently published ‘How to do a 3-minute diabetic foot exam’ as a screening test for detecting sensory loss [143]. This has also recently been approved by the American Diabetes Association (Prof. A Boulton, presentation at the ADA Scientific meeting, Boston 2015).
4. Validation of normative values
Normative values are data that characterize, in a pre-defined population, what is usual at a time point or period of time for a specific test or technique [144]. Such data are very useful in diabetic neuropathy as understanding normative change (related to morphology and function) allows for defining the presence or absence of the condition, and provides useful insight into the possible etiopathogenesis. However, in studies designed to obtain normative data, the analytical sample should be rigorously selected from clear predefined criteria; the methodology should be robust and reproducible, and the interpretation of the results should be appropriate [144-145]. Furthermore, when age effects are to be described or when time is an important consideration, then longitudinal study designs may be needed to evaluate potential cohort effects and epoch effects [144].
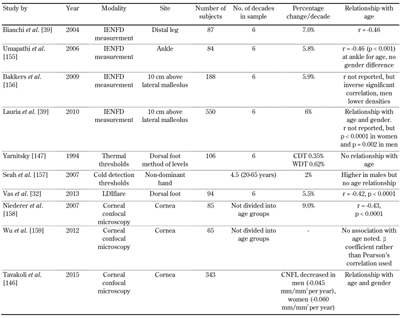
Both the somatic and autonomic peripheral nervous system change with age [1, 32, 39, 146]. It is therefore important that accurate normative data is determined with modern sensitive techniques developed for the detection of DSPN. An excellent example is the worldwide normative data report by Lauria and colleagues from Europe and North America for IENFD [39]. Apart from age- and gender-specific normative values, the paper also standardizes the protocol used to derive such data. A similar endeavor with the CCM, published recently, has pooled 1,965 images form 343 healthy volunteers across Europe, Australia, and North America, allowing practical and single-protocol-driven use of this technique [146].
While the commercial tests for thermal thresholds and sudomotor testing have established large normative databases [14, 147-149], tests for small fiber function such as LDIflare and CHEPS have recently established age-defined centile charts [32, 105]. There are no current normative values for microneurography. It is important to note that normative data currently available for these select tests are for predominant European/Caucasian cohorts; their application in other ethnicities has not been fully studied. We have summarized a selection of the available normative data for small fiber tests (Table 6).
Table
6.
Selection of the normative data available |
|
 |
 |
Legend:
CDT – cold detection threshold; CNFL – corneal nerve fiber length; IENFD – intraepidermal nerve fiber density; LDIflare – laser Doppler imager flare; WDT – warm detection threshold. |
|
A recent study has shown that the use of age-based normative values may enhance the diagnostic efficacy of the test [32]. However, there exists considerable variation in methodology used by research teams for the same diagnostic technique, an issue that affects normative results and diagnostic accuracy [150].
5. Genetic studies in DSPN
A significant body of new evidence is pointing towards a link between genetic factors and the development of diabetic complications [151]. Studies have reported risk association for the genes coding for factors such as vascular endothelial growth factor (VGEF) in retinopathy, engulfment and cell motility (ELMO1) in nephropathy, and ADIPOQ in coronary artery disease [151]. Candidate gene studies of dysglycemic pathways have uncovered genes coding for aldose reductase activity inhibition (such as AKR1 B1) in the development of diabetic retinopathy and nephropathy [151-152]. Aldose reductase is a key rate-limiting enzyme in the polyol pathway also implicated in the development of diabetic neuropathy.
Studies looking at small fiber neuropathic pain have also established roles of various sodium channels (gain of function SCN9A mutations), while mutations in the vanniloid receptor gene, TRPA1, may lead to episodic familial pain syndromes [31]. Similarly, channelopathies leading to loss of function mutations may cause insensitivity to pain syndromes [153]. Polymorphisms in the adiponectin (ADPN) gene, T45G and G276T, have also recently been associated with increased risk of developing DSPN in type 2 diabetes [154]. The authors concluded that the polymorphisms led to a downregulation of ADPN serum level, an insulin sensitizer and anti-inflammatory agent. However, the reported associations were weak and inconsistent, and no studies predicted a clear relationship of any specific genetic mono- or polymorphism with DSPN. Nevertheless, this is an exciting field of future research, both from a therapeutic and a clinical perspective.
6. Summary
DSPN is a heterogeneous constellation of clinical and subclinical syndromes. The development of modern techniques able to more precisely measure function and structure of small fibers has led to earlier detection and better characterization of this condition. Nevertheless, the field is still in its infancy, and the ability of some of the modalities to demonstrate neuronal regeneration, whilst exciting, needs to be evaluated as a proof of principle.
Furthermore, etiopathogenesis studies are relatively sparse and studies of neuronal plasticity and regeneration, in particular potential differences between the young and aged, are lacking. Large fiber markers are being refined constantly, and the availability of a point-of-care device may reinvent screening for neuropathy. At the same time, the availability of new methods to detect loss of protective sensation, some of them with no cost implications, enables neuropathy screening to all those with diabetes, in particular in regions of the world with resource limitations.
Disclosures: The authors report no conflict of interests.
References
- Said G. Diabetic neuropathy - a review. Nat Clin Pract Neuro 2007. 3(6):331-340. [DOD] [CrossRef]
- Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003. 26(6):1895-1901. [DOD] [CrossRef]
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005. 28(4):956-962. [DOD] [CrossRef]
- Dyck PJ, Melton LJ 3rd, O'Brien PC, Service FJ. Approaches to improve epidemiological studies of diabetic neuropathy: insights from the Rochester Diabetic Neuropathy Study. Diabetes 1997. 46(Suppl 2):S5-S8. [DOD] [CrossRef]
- Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J 2007. 4(4):286-287. [DOD]
- Boulton AJ, Malik RA. Neuropathy of impaired glucose tolerance and its measurement. Diabetes Care 2010. 33(1):207-209. [DOD] [CrossRef]
- Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes 1997. 46(Suppl 2):S54-S57. [DOD] [CrossRef]
- England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005. 64(2):199-207. [DOD] [CrossRef]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010. 33(10):2285-2293. [DOD] [CrossRef]
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993. 36(2):150-154. [DOD] [CrossRef]
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002. 19(5):377-384. [DOD] [CrossRef]
- Papanas N, Papatheodorou K, Papazoglou D, Kotsiou S, Maltezos E. A prospective study on the use of the indicator test Neuropad(R) for the early diagnosis of peripheral neuropathy in type 2 diabetes. Exp Clin Endocrinol Diabetes 2011. 119(2):122-125. [DOD] [CrossRef]
- Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol 2010. 223(1):245-250. [DOD] [CrossRef]
- Dyck PJ, Bushek W, Spring EM, Karnes JL, Litchy WJ, O'Brien PC, Service FJ. Vibratory and cooling detection thresholds compared with other tests in diagnosing and staging diabetic neuropathy. Diabetes Care 1987. 10(4):432-440. [DOD] [CrossRef]
- Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve 1988. 11(1):21-32. [DOD] [CrossRef]
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994. 17(11):1281-1289. [DOD] [CrossRef]
- Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, Feldman EL, DCCT/EDIC Research Group. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012. 29(7):937-944. [DOD] [CrossRef]
- Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002. 25(11):2048-2052. [DOD] [CrossRef]
- Bril V, Tomioka S, Buchanan RA, Perkins BA. Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 2009. 26(3):240-246. [DOD] [CrossRef]
- Zilliox LA, Ruby SK, Singh S, Zhan M, Russell JW. Clinical neuropathy scales in neuropathy associated with impaired glucose tolerance. J Diabetes Complications. 29(3):372-377. [DOD] [CrossRef]
- Perkins BA, Bril V. Diabetic neuropathy: a review emphasizing diagnostic methods. Clin Neurophysiol 2003. 114(7):1167-1175. [DOD] [CrossRef]
- Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med 2002. 19(11):962-965. [DOD] [CrossRef]
- Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology 1997. 49(1):229-239. [DOD] [CrossRef]
- Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, Howard J, Smith AG. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst 2008. 13(3):218-227. [DOD] [CrossRef]
- Gordon Smith A. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012. 17(Suppl 2):15-21. [DOD] [CrossRef]
- Green AQ, Krishnan S, Finucane FM, Rayman G. Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care 2010. 33(1):174-176. [DOD] [CrossRef]
- Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol 2011. 7(11):682-690. [DOD] [CrossRef]
- Breiner A, Lovblom LE, Perkins BA, Bril V. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014. 37(5):1418-1424. [DOD]
- Lauria G. Small fibre neuropathies. Curr Opin Neurol 2005. 18(5):591-597. [DOD] [CrossRef]
- Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, Broglio L, Granieri E, Lauria G. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008. 131(Pt 7):1912-1925. [DOD] [CrossRef]
- Themistocleous AC, Ramirez JD, Serra J, Bennett DL. The clinical approach to small fibre neuropathy and painful channelopathy. Pract Neurol 2014. 14(6):368-379. [DOD] [CrossRef]
- Vas PR, Rayman G. The rate of decline in small fibre function assessed using axon reflex-mediated neurogenic vasodilatation and the importance of age related centile values to improve the detection of clinical neuropathy. Plos One 2013. 8(7):e69920. [DOD] [CrossRef]
- Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology 2009. 54(3):273-285. [DOD] [CrossRef]
- Petropoulos IN, Green P, Chan AW, Alam U, Fadavi H, Marshall A, Asghar O, Efron N, Tavakoli M, Malik RA. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. Plos One 2015. 10(4):e0123517. [DOD] [CrossRef]
- Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care 2015. 38(4):671-675. [DOD]
- Dehghani C, Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, Efron N. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci 2014. 55(12):7982-7990. [DOD] [CrossRef]
- McCarthy B, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath D, Griffin J, McArthur J. Cutaneous innervation in sensory neuropathies evaluation by skin biopsy. Neurology 1995. 45(10):1848-1855. [DOD] [CrossRef]
- Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010. 17(7):903-912. [DOD] [CrossRef]
- Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010. 15(3):202-207. [DOD] [CrossRef]
- Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010. 17(7):903-912, e944-909. [DOD] [CrossRef]
- Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology 1996. 47(4):1042-1048. [DOD] [CrossRef]
- Nebuchennykh M, Loseth S, Lindal S, Mellgren SI. The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J Neurol 2009. 256(7):1067-1075. [DOD] [CrossRef]
- Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, Singleton JR. Lifestyle Intervention for Pre-Diabetic Neuropathy. Diabetes Care 2006. 29(6):1294-1299. [DOD] [CrossRef]
- Singleton JR, Marcus RL, Jackson JE, Lessard MK, Graham TE, Smith AG. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol 2014. 1(10):844-849. [DOD] [CrossRef]
- Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol 2015. 77(1):146-153. [DOD] [CrossRef]
- Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, Boulton AJ. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003. 46(5):683-688. [DOD]
- Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007. 56(8):2148-2154. [DOD] [CrossRef]
- Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, Morgan P, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010. 33(8):1792-1797. [DOD] [CrossRef]
- Petropoulos IN, Alam U, Fadavi H, Asghar O, Green P, Ponirakis G, Marshall A, Boulton AJ, Tavakoli M, Malik RA. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care 2013. 36(11):3646-3651. [DOD] [CrossRef]
- Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, Orlov S, Perkins BA. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care 2012. 35(4):821-828. [DOD] [CrossRef]
- Tavakoli M, Kallinikos P, Iqbal A, Herbert A, Fadavi H, Efron N, Boulton AJ, Malik R. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med 2011. 28(10):1261-1267. [DOD] [CrossRef]
- Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care 2007. 30(10):2608-2612. [DOD] [CrossRef]
- Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, Ponirakis G, Jeziorska M, Marshall A, Efron N, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 2013. 62(1):254-260. [DOD] [CrossRef]
- Azmi S, Ferdousi M, Petropoulos IN, Ponirakis G, Fadavi H, Tavakoli M, Alam U, Jones W, Marshall A, Jeziorska M, et al. Corneal confocal microscopy shows an improvement in small-fiber neuropathy in subjects with type 1 diabetes on continuous subcutaneous insulin infusion compared with multiple daily injection. Diabetes Care 2015. 38(1):e3-e4. [DOD] [CrossRef]
- Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal 2011. 15(5):738-747. [DOD] [CrossRef]
- Dabbah MA, Graham J, Petropoulos I, Tavakoli M, Malik RA. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med Image Comput Comput Assist Interv 2010. 13(Pt 1):300-307. [DOD]
- Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, Alam U, Marshall A, Fadavi H, Ferdousi M, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015. 38(6):1138-1144. [DOD] [CrossRef]
- Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Bruggemann J, Strom A, Peschel S, Kohler B, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014. 63(7):2454-2463. [DOD] [CrossRef]
- Azmi S, Ferdousi M, Petropoulos IN, Ponirakis G, Alam U, Fadavi H, Asghar O, Marshall A, Atkinson AJ, Jones W, et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care 2015. In press.[DOD]
- Bickel A, Kramer HH, Hilz MJ, Birklein F, Neundorfer B, Schmelz M. Assessment of the neurogenic flare reaction in small-fiber neuropathies. Neurology 2002. 59(6):917-919. [DOD] [CrossRef]
- Kramer HH, Schmelz M, Birklein F, Bickel A. Electrically stimulated axon reflexes are diminished in diabetic small fiber neuropathies. Diabetes 2004. 53(3):769-774. [DOD] [CrossRef]
- Bickel A, Heyer G, Senger C, Maihofner C, Heuss D, Hilz MJ, Namer B. C-fiber axon reflex flare size correlates with epidermal nerve fiber density in human skin biopsies. J Peripher Nerv Syst 2009. 14(4):294-299. [DOD] [CrossRef]
- Hamdy O, Abou-Elenin K, LoGerfo FW, Horton ES, Veves A. Contribution of nerve-axon reflex-related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care 2001. 24(2):344-349. [DOD] [CrossRef]
- Krishnan ST, Rayman G. The LDIflare A novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care 2004. 27(12):2930-2935. [DOD] [CrossRef]
- Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Abnormal LDIflare but normal quantitative sensory testing and dermal nerve fiber density in patients with painful diabetic neuropathy. Diabetes Care 2009. 32(3):451-455. [DOD] [CrossRef]
- Vas PR, Sharma S, Rayman G. LDIflare small fiber function in normal glucose tolerant subjects with and without hypertriglyceridemia. Muscle Nerve 2014. 52(1):113-119. [DOD] [CrossRef]
- Vas PR, Green AQ, Rayman G. Small fibre dysfunction, microvascular complications and glycaemic control in type 1 diabetes: a case-control study. Diabetologia 2012. 55(3):795-800. [DOD] [CrossRef]
- Nabavi Nouri M, Ahmed A, Bril V, Orszag A, Ng E, Nwe P, Perkins BA. Diabetic neuropathy and axon reflex-mediated neurogenic vasodilatation in type 1 diabetes. Plos One 2012. 7(4):e34807. [DOD] [CrossRef]
- Vas PR, Rayman G. Validation of the modified LDIFlare technique: a simple and quick method to assess C-fiber function. Muscle Nerve 2013. 47(3):351-356. [DOD] [CrossRef]
- Sharma S, Vas PR, Rayman G. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol 2015. 9(1):123-131. [DOD] [CrossRef]
- Kennedy WR, Navarro X. Sympathetic sudomotor function in diabetic neuropathy. Arch Neurol 1989. 46(11):1182-1186. [DOD] [CrossRef]
- Gin H, Baudoin R, Raffaitin CH, Rigalleau V, Gonzalez C. Non-invasive and quantitative assessment of sudomotor function for peripheral diabetic neuropathy evaluation. Diabetes Metab 2011. 37(6):527-532. [DOD] [CrossRef]
- Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle Nerve 2013. 48(6):883-888. [DOD] [CrossRef]
- Riedel A, Braune S, Kerum G, Schulte-Monting J, Lucking CH. Quantitative sudomotor axon reflex test (QSART): a new approach for testing distal sites. Muscle Nerve 1999. 22(9):1257-1264. [DOD] [CrossRef]
- Shimada H, Kihara M, Kosaka S, Ikeda H, Kawabata K, Tsutada T, Miki T. Comparison of SSR and QSART in early diabetic neuropathy - the value of length-dependent pattern in QSART. Auton Neurosci 2001. 92(1-2):72-75. [DOD] [CrossRef]
- Sletten DM, Weigand SD, Low PA. Relationship of Q-sweat to quantitative sudomotor axon reflex test (QSART) volumes. Muscle Nerve 2010. 41(2):240-246. [DOD]
- Shahani BT, Halperin J, Boulu P, Cohen J. Sympathetic skin response - a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry 1984. 47(5):536-542. [DOD] [CrossRef]
- Braune HJ, Horter C. Sympathetic skin response in diabetic neuropathy: a prospective clinical and neurophysiological trial on 100 patients. J Neurol Sci 1996. 138(1-2):120-124. [DOD] [CrossRef]
- Papanas N, Papatheodorou K, Christakidis D, Papazoglou D, Giassakis G, Piperidou H, Monastiriotis C, Maltezos E. Evaluation of a new indicator test for sudomotor function (Neuropad) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes 2005. 113(4):195-198. [DOD] [CrossRef]
- Papanas N, Giassakis G, Papatheodorou K, Papazoglou D, Monastiriotis C, Christakidis D, Piperidou H, Maltezos E. Sensitivity and specificity of a new indicator test (Neuropad) for the diagnosis of peripheral neuropathy in type 2 diabetes patients: a comparison with clinical examination and nerve conduction study. J Diabetes Complications 2007. 21(6):353-358. [DOD] [CrossRef]
- Ponirakis G, Petropoulos IN, Fadavi H, Alam U, Asghar O, Marshall A, Tavakoli M, Malik RA. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet Med 2014. 31(12):1673-1680. [DOD] [CrossRef]
- Manes C, Papanas N, Exiara T, Katsiki N, Papantoniou S, Kirlaki E, Tsotoulidis S, Kefalogiannis N, Maltezos E. The indicator test Neuropad in the assessment of small and overall nerve fibre dysfunction in patients with type 2 diabetes: a large multicentre study. Exp Clin Endocrinol Diabetes 2014. 122(3):195-199. [DOD] [CrossRef]
- Quattrini C, Jeziorska M, Tavakoli M, Begum P, Boulton AJ, Malik RA. The Neuropad test: a visual indicator test for human diabetic neuropathy. Diabetologia 2008. 51(6):1046-1050. [DOD] [CrossRef]
- Ponirakis G, Fadavi H, Petropoulos IN, Azmi S, Ferdousi M, Dabbah MA, Kheyami A, Alam U, Asghar O, Marshall A, et al. Automated quantification of Neuropad improves its diagnostic ability in patients with diabetic neuropathy. J Diabetes Res 2015. 2015:847854. [DOD] [CrossRef]
- Yoshioka K, Okada H. Useful application of the Neuropad test for assessment of diabetic polyneuropathy. Intern Med 2012. 51(23):3241-3245. [DOD] [CrossRef]
- Kamenov ZA, Petrova JJ, Christov VG. Diagnosis of diabetic neuropathy using simple somatic and a new autonomic (Neuropad) tests in the clinical practice. Exp Clin Endocrinol Diabetes 2010. 118(4):226-233. [DOD] [CrossRef]
- Korei AE, Istenes I, Papanas N, Kempler P. Small-fiber neuropathy: a diabetic microvascular complication of special clinical, diagnostic, and prognostic importance. Angiology 2015. In press. [DOD]
- Mayaudon H, Miloche PO, Bauduceau B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab 2010. 36(6 Pt 1):450-454. [DOD] [CrossRef]
- Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 2013. 15(11):948-953. [DOD] [CrossRef]
- Yang Z, Xu B, Lu J, Tian X, Li M, Sun K, Huang F, Liu Y, Xu M, Bi Y, Wang W. Autonomic test by EZSCAN in the screening for prediabetes and diabetes. Plos One 2013. 8(2):e56480. [DOD] [CrossRef]
- Chen L, Chen X, Ding R, Shi Q Jr, Hu D. Evaluation of EZSCAN as a screening tool for impaired glucose metabolism. Diabetes Res Clin Pract 2013. 100(2):210-214. [DOD] [CrossRef]
- Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications 2014. 28(4):511-516. [DOD] [CrossRef]
- Report and Recommendations of the San Antonio Conference on Diabetic Neuropathy. Diabetes 1988. 37(7):1000-1004. [DOD] [CrossRef]
- Viswanathan V, Snehalatha C, Seena R, Ramachandran A. Early recognition of diabetic neuropathy: evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J 2002. 78(923):541-542. [DOD] [CrossRef]
- Peltier A, Smith AG, Russell JW, Sheikh K, Bixby B, Howard J, Goldstein J, Song Y, Wang L, Feldman EL, Singleton JR. Reliability of quantitative sudomotor axon reflex testing and quantitative sensory testing in neuropathy of impaired glucose regulation. Muscle Nerve 2009. 39(4):529-535. [DOD] [CrossRef]
- Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giuliani MJ, Kincaid JC, Ochoa JL, Parry GJ, Weimer LH. Quantitative sensory testing: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003. 60(6):898-904. [DOD] [CrossRef]
- Geber C, Klein T, Azad S, Birklein F, Gierthmühlen J, Huge V, Lauchart M, Nitzsche D, Stengel M, Valet M, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): A multi-centre study. Pain 2011. 152(3):548-556. [DOD] [CrossRef]
- Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, Haanpaa M, Jensen TS, Serra J, Treede RD. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol 2010. 17(8):1010-1018. [DOD] [CrossRef]
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013. 154(9):1807-1819. [DOD] [CrossRef]
- Klima RR, Weigand AH, DeLisa JA. Nerve conduction studies and vibration perception thresholds in diabetic and uremic neuropathy. Am J Phys Med Rehabil 1991. 70(2):86-90. [DOD] [CrossRef]
- Bril V, Perkins BA. Comparison of vibration perception thresholds obtained with the Neurothesiometer and the CASE IV and relationship to nerve conduction studies. Diabet Med 2002. 19(8):661-666. [DOD] [CrossRef]
- Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care 1994. 17(6):557-560. [DOD] [CrossRef]
- Tsui BH, Shakespeare T, Leung D, Tsui J, Corry G. Reproducibility of current perception threshold with the Neurometer vs. the Stimpod NMS450 peripheral nerve stimulator in healthy volunteers: an observational study. Can J Anaesth 2013. 60(8):753-760. [DOD] [CrossRef]
- Atherton DD, Facer P, Roberts KM, Misra VP, Chizh BA, Bountra C, Anand P. Use of the novel Contact Heat Evoked Potential Stimulator (CHEPS) for the assessment of small fibre neuropathy: correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol 2007. 7:21. [DOD] [CrossRef]
- Lagerburg V, Bakkers M, Bouwhuis A, Hoeijmakers JG, Smit AM, Van Den Berg SJ, Hordijk-De Boer I, Brouwer-Van Der Lee MD, Kranendonk D, Reulen JP, et al. Contact heat evoked potentials: normal values and use in small-fiber neuropathy. Muscle Nerve 2015. 51(5):743-749. [DOD] [CrossRef]
- Serra J. Re-emerging microneurography. J Physiol 2009. 587(Pt 2):295-296. [DOD] [CrossRef]
- Schmelz M, Forster C, Schmidt R, Ringkamp M, Handwerker HO, Torebjörk HE. Delayed responses to electrical stimuli reflect C-fiber responsiveness in human microneurography. Experimental Brain Research 1995. 104(2):331-336. [DOD] [CrossRef]
- Rice AS, Andreev NY, McMahon SB. The consequences of microneurography electrode-induced injury of peripheral nerves observed in the rat and man. Pain 1994. 59(3):385-393. [DOD] [CrossRef]
- Namer B, Barta B, Orstavik K, Schmidt R, Carr R, Schmelz M, Handwerker HO. Microneurographic assessment of C-fibre function in aged healthy subjects. J Physiol 2009. 587(Pt 2):419-428. [DOD] [CrossRef]
- Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011. 27(7):639-653. [DOD] [CrossRef]
- Tobin K, Giuliani MJ, Lacomis D. Comparison of different modalities for detection of small fiber neuropathy. Clin Neurophysiol 1999. 110(11):1909-1912. [DOD] [CrossRef]
- Softeland E, Brock C, Frokjaer JB, Brogger J, Madacsy L, Gilja OH, Arendt-Nielsen L, Simren M, Drewes AM, Dimcevski G. Association between visceral, cardiac and sensorimotor polyneuropathies in diabetes mellitus. J Diabetes Complications 2014. 28(3):370-377. [DOD] [CrossRef]
- Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007. 115(3):387-397. [DOD] [CrossRef]
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992. 15(6):661-665. [DOD] [CrossRef]
- American Diabetes Association. 9. Microvascular Complications and Foot Care. Diabetes Care 2015. 38(Suppl 1):S58-S66. [DOD] [CrossRef]
- Booth J, Young MJ. Differences in the performance of commercially available 10-g monofilaments. Diabetes Care 2000. 23(7):984-988. [DOD] [CrossRef]
- Chaudhry V, Cornblath DR, Mellits ED, Avila O, Freimer ML, Glass JD, Reim J, Ronnett GV, Quaskey SA, Kuncl RW. Inter- and intra-examiner reliability of nerve conduction measurements in normal subjects. Ann Neurol 1991. 30(6):841-843. [DOD] [CrossRef]
- Gilliatt RW. Experimental peripheral neuropathy. Sci Basis Med Annu Rev. 1969:203-219. [DOD]
- Thomas PK. Diabetic neuropathy: morphological aspects. Proc R Soc Med 1967. 60(2):145-145. [DOD]
- Dyck PJ, Karnes JL, Daube J, O'Brien P, Service FJ. Clinical and neuropathological criteria for the diagnosis and staging of diabetic polyneuropathy. Brain 1985. 108(4):861-880. [DOD] [CrossRef]
- Dyck PJ, Lais A, Karnes JL, O'Brien P, Rizza R. Fiber loss is primary and multifocal in sural nerves in diabetic polyneuropathy. Ann Neurol 1986. 19(5):425-439. [DOD] [CrossRef]
- Dyck PJ, Carter RE, Litchy WJ. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve 2011. 44(3):340-345. [DOD]
- Boulton AJ. Whither clinical research in diabetic sensorimotor peripheral neuropathy? Problems of end point selection for clinical trials. Diabetes Care 2007. 30(10):2752-2753. [DOD] [CrossRef]
- Rull J, Quibrera R, Gonzalez-Millan H, Castaneda OL. Symptomatic treatment of peripheral diabetic neuropathy with carbamazepine Tegretol: double blind crossover trial. Diabetologia 1969. 5(4):215-218. [DOD] [CrossRef]
- Benatar M, Chapman KM, Rutkove SB. Repetitive nerve stimulation for the evaluation of peripheral nerve hyperexcitability. J Neurol Sci 2004. 221(1):47-52. [DOD] [CrossRef]
- Harrison TB, Benatar M. Accuracy of repetitive nerve stimulation for diagnosis of the cramp–fasciculation syndrome. Muscle Nerve 2007. 35(6):776-780. [DOD] [CrossRef]
- Kohara N, Kimura J, Kaji R, Goto Y, Ishii J, Takiguchi M, Nakai M. F-wave latency serves as the most reproducible measure in nerve conduction studies of diabetic polyneuropathy: multicentre analysis in healthy subjects and patients with diabetic polyneuropathy. Diabetologia 2000. 43(7):915-921. [DOD] [CrossRef]
- Andersen H, Stalberg E, Falck B. F‐wave latency, the most sensitive nerve conduction parameter in patients with diabetes mellitus. Muscle Nerve 1997. 20(10):1296-1302. [DOD] [CrossRef]
- Dunnigan SK, Ebadi H, Breiner A, Katzberg HD, Barnett C, Perkins BA, Bril V. The characteristics of chronic inflammatory demyelinating polyneuropathy in patients with and without diabetes - an observational study. Plos One 2014. 9(2):e89344. [DOD] [CrossRef]
- Litchy WJ, Albers JW, Wolfe J, Bolton CF, Walsh N, Klein CJ, Zafft AJ, Russell JW, Zwirlein M, Overland CJ, et al. Proficiency of nerve conduction using standard methods and reference values (cl. NPhys Trial 4). Muscle Nerve 2014. 50(6):900-908. [DOD] [CrossRef]
- Albers JW, Brown MB, Sima AA, Greene DA. Nerve conduction measures in mild diabetic neuropathy in the Early Diabetes Intervention Trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Neurology 1996. 46(1):85-91. [DOD] [CrossRef]
- Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O'Brien PC. Variables influencing neuropathic endpoints: The Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology 1995. 45(6):1115-1121. [DOD] [CrossRef]
- Perkins BA, Grewal J, Ng E, Ngo M, Bril V. Validation of a novel point-of-care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care 2006. 29(9):2023-2027. [DOD] [CrossRef]
- Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and Validity of a Point-of-Care Sural Nerve Conduction Device for Identification of Diabetic Neuropathy. Plos One 2014. 9(1):e86515. [DOD] [CrossRef]
- Perkins BA, Orszag A, Grewal J, Ng E, Ngo M, Bril V. Multi-site testing with a point-of-care nerve conduction device can be used in an algorithm to diagnose diabetic sensorimotor polyneuropathy. Diabetes Care 2008. 31(3):522-524. [DOD] [CrossRef]
- Willits I, Cole H, Jones R, Dimmock P, Arber M, Craig J, Sims A. VibraTip for Testing Vibration Perception to Detect Diabetic Peripheral Neuropathy: A NICE Medical Technology Guidance. Appl Health Econ Health Policy 2015. In press. [DOD]
- Bowling FL, Abbott CA, Harris WE, Atanasov S, Malik RA, Boulton AJ. A pocket-sized disposable device for testing the integrity of sensation in the outpatient setting. Diabet Med 2012. 29(12):1550-1552. [DOD] [CrossRef]
- Bracewell N, Game F, Jeffcoate W, Scammell BE. Clinical evaluation of a new device in the assessment of peripheral sensory neuropathy in diabetes. Diabet Med 2012. 29(12):1553-1555. [DOD] [CrossRef]
- Nizar H, Munro N, Nightingale P, Fehrer MD. Diagnostic accuracy of the VibraTip in detection of diabetic peripheral neuropathy. Brit J Diabetes Vasc Dis 2014. 14:26-29. [DOD] [CrossRef]
- Rayman G, Vas PR, Baker N, Taylor CG Jr, Gooday C, Alder AI, Donohoe M. The Ipswich Touch Test: a simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care 2011. 34(7):1517-1518. [DOD] [CrossRef]
- Sharma S, Kerry C, Atkins H, Rayman G. The Ipswich Touch Test: a simple and novel method to screen patients with diabetes at home for increased risk of foot ulceration. Diabet Med 2014. 31(9):1100-1103. [DOD] [CrossRef]
- Madanat A, Sheshah E, Badawy EB, Abbas A, Al-Bakheet A. Utilizing the Ipswich Touch Test to simplify screening methods for identifying the risk of foot ulceration among diabetics: The Saudi experience. Prim Care Diabetes 2014. 9(4):304-306. [DOD] [CrossRef]
- Miller JD, Carter E, Shih J, Giovinco NA, Boulton AJ, Mills JL, Armstrong DG. How to do a 3-minute diabetic foot exam. J Fam Pract 2014. 63(11):646-656. [DOD]
- O'Connor PJ. Normative data: their definition, interpretation, and importance for primary care physicians. Fam Med 1990. 22(4):307-311. [DOD]
- Schmidt S, Pardo Y. Normative data. Encycl Qual Life Well-Being Res 2014. 2014:4375-4379. [DOD]
- Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, Ziegler D, Pacaud D, Romanchuk K, Perkins BA, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care 2015. 38(5):838-843. [DOD] [CrossRef]
- Yarnitsky D, Sprecher E. Thermal testing: normative data and repeatability for various test algorithms. J Neurol Sci 1994. 125(1):39-45. [DOD] [CrossRef]
- Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997. 20(12):1561-1568. [DOD] [CrossRef]
- Sletten D, Grandinetti A, Weigand S, Gehrking T, Gehrking J, Low P, Singer W. Normative values for sudomotor axon reflex testing using QSWEAT (P1.282). Neurology 2015. 84(14 Supplement). [DOD]
- Vas PR, Sharma S, Rayman G. Comment on Breiner et al. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014. 37(11):e240-e241. [DOD]
- Doria A. Genetics of Diabetes Complications. Curr Diabetes Rep 2010. 10(6):467-475. [DOD] [CrossRef]
- Donaghue KC, Margan SH, Chan AK, Holloway B, Silink M, Rangel T, Bennetts B. The association of aldose reductase gene (AKR1B1) polymorphisms with diabetic neuropathy in adolescents. Diabet Med 2005. 22(10):1315-1320. [DOD] [CrossRef]
- Nilsen KB, Nicholas AK, Woods CG, Mellgren SI, Nebuchennykh M, Aasly J. Two novel SCN9A mutations causing insensitivity to pain. Pain 2009. 143(1-2):155-158. [DOD] [CrossRef]
- Ji ZY, Li HF, Lei Y, Rao YW, Tan ZX, Liu HJ, Yao GD, Hou B, Sun ML. Association of adiponectin gene polymorphisms with an elevated risk of diabetic peripheral neuropathy in type 2 diabetes patients. J Diabetes Complications 2015. In press.[DOD]
- Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve 2007. 35(5):591-598. [DOD] [CrossRef]
- Bakkers M, Merkies IS, Lauria G, Devigili G, Penza P, Lombardi R, Hermans MC, van Nes SI, De Baets M, Faber CG. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009. 73(14):1142-1148. [DOD] [CrossRef]
- Seah SA, Griffin MJ. Normal values for thermotactile and vibrotactile thresholds in males and females. Int Arch Occup Environ Health 2008. 81(5):535-543. [DOD] [CrossRef]
- Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol 2007. 91(9):1165-1169. [DOD] [CrossRef]
- Wu T, Ahmed A, Bril V, Orszag A, Ng E, Nwe P, Perkins BA. Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med 2012. 29(9):e297-e303. [DOD] [CrossRef]
This article has been cited by other articles:
|