Chapter II. Diabetic Nephropathy
| Rev Diabet Stud,
2015,
12(1-2):110-118 |
DOI 10.1900/RDS.2015.12.110 |
Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis
Konstantinos Tziomalos1, Vasilios G. Athyros2
1First Propedeutic Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, AHEPA Hospital, Thessaloniki, Greece
2Second Propedeutic Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, Hippokration Hospital, Thessaloniki, Greece
Address correspondence to: Konstantinos Tziomalos, First Propedeutic Department of Internal Medicine, Aristotle University of Thessaloniki, AHEPA Hospital, Thessaloniki 54636, Greece, e-mail: ktziomalos@yahoo.com
Manuscript submitted April 10, 2015; resubmitted April 30, 2015; accepted April 30, 2015.
Keywords: diabetic nephropathy, albuminuria, glomerular filtration rate, diagnosis, risk factors, diabetes
Abstract
Diabetic nephropathy is the leading cause of end-stage renal disease. Patients with diabetic nephropathy have a high cardiovascular risk, comparable to patients with coronary heart disease. Accordingly, identification and management of risk factors for diabetic nephropathy as well as timely diagnosis and prompt management of the condition are of paramount importance for effective treatment. A variety of risk factors promotes the development and progression of diabetic nephropathy, including elevated glucose levels, long duration of diabetes, high blood pressure, obesity, and dyslipidemia. Most of these risk factors are modifiable by antidiabetic, antihypertensive, or lipid-lowering treatment and lifestyle changes. Others such as genetic factors or advanced age cannot be modified. Therefore, the rigorous management of the modifiable risk factors is essential for preventing and delaying the decline in renal function. Early diagnosis of diabetic nephropathy is another essential component in the management of diabetes and its complications such as nephropathy. New markers may allow earlier diagnosis of this common and serious complication, but further studies are needed to clarify their additive predictive value, and to define their cost-benefit ratio. This article reviews the most important risk factors in the development and progression of diabetic nephropathy and summarizes recent developments in the diagnosis of this disease.
Abbreviations: 8-oxodG – 8-oxo-7,8-dihydro-2’-deoxyguanosine; ACE – angiotensin-converting enzyme; ADA – American Diabetes Association; CKD-EPI – Chronic Kidney Disease Epidemiology Collaboration; DCCT/EDIC – Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications; ESRD – end-stage renal disease; GFR – glomerular filtration rate; GWAS - genome-wide association studies; HbA1c – glycosylated hemoglobin; hsCRP - high-sensitivity C-reactive protein; KIM-1 – kidney injury molecule 1; LDL-C – low-density lipoprotein cholesterol; MDRD – Modified Diet in Renal Disease; NAG – N-acetyl-β-D-glucosaminidase; T1D – type 1 diabetes; T2D – type 2 diabetes; TC – total cholesterol; TG - triglyceride; UAE – urinary albumin excretion
1. Introduction
The prevalence of type 2 diabetes (T2D) is increasing worldwide as a consequence of the rising number of obese patients [1]. Diabetic nephropathy affects approximately 25% of patients with T2D, and represents the leading cause of end-stage renal disease (ESRD) in high-income countries [2, 3]. Moreover, patients with diabetic nephropathy have very high cardiovascular risk, which is comparable with the cardiovascular risk of patients with coronary heart disease [4, 5]. Treatment with statins, angiotensin converting enzyme (ACE) inhibitors, or angiotensin receptor blockers delays renal disease progression and reduces cardiovascular morbidity in these patients [6-14], whereas antidiabetic treatment prevents diabetic nephropathy and delays the progression of diabetic nephropathy [15-21]. Accordingly, identification and management of risk factors for diabetic nephropathy as well as timely diagnosis and prompt management of the disease are of paramount importance.
In the present review, we discuss the most important risk factors for development and progression of diabetic nephropathy, and we summarize recent developments in the diagnosis of this disease.
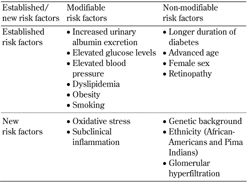
2. Risk factors for diabetic nephropathy (Table 1)
2.1 Increased urinary albumin excretion
Increased urinary albumin excretion is a major risk factor for the progression of diabetic nephropathy in both type 1 diabetes (T1D) and T2D [22-24]. In most patients, the first sign of diabetic nephropathy is moderately increased urinary albumin excretion, i.e. 30-300 mg/g creatinine in a spot urine sample (also termed microalbuminuria) [25]. Patients who develop severely increased albuminuria, i.e. >300 mg albumin/g creatinine in a spot urine sample (also called macroalbuminuria or clinical albuminuria), are at particularly high risk for developing a decline in renal function [16, 25-27].
However, a substantial proportion (up to 40%) of patients with moderate albuminuria returns to normoalbuminuria [16, 28, 29]. Moreover, up to 50% of patients with T1D or T2D experience a decline in glomerular filtration rate (GFR), despite the presence of only moderate albuminuria or even normoalbuminuria [29-32]. Therefore, elevated urinary albumin excretion (UAE) is not a necessary prerequisite for the development of diabetic nephropathy. This finding has consequences for the diagnosis of the disease, namely that GFR should be assessed in addition to UAE. Moreover, elevated UAE is associated with increased cardiovascular risk, but it is controversial whether reducing it translates to a lower incidence of cardiovascular events.
Table
1.
Risk factors for diabetic nephropathy |
|
 |
 |
2.2 Elevated glucose levels
Inadequate glycemic control is a pivotal risk factor for the development and progression of diabetic nephropathy. In patients with both T1D and T2D, high HbA1c levels are associated with an increased risk for developing nephropathy [24, 28, 33, 34]. Observational studies reported a clear decrease in the incidence of diabetic nephropathy in both patients with T1D and T2D who achieved better glycemic control [16, 35, 36]. Indeed, in the Diabetes Control and Complications Trial/Epi-demiology of Diabetes Interventions and Complications (DCCT/EDIC) study, patients with moderate albuminuria, but lower HbA1c levels, had a lower risk for progressing to severe albuminuria or ESRD [16]. Randomized controlled studies in patients with T1D and T2D reported similar findings [15-21]. In DCCT, intensive glycemic control reduced the risk of progression from moderate albuminuria to severe albuminuria or ESRD [16]. Moreover, strict glycemic control reduces the risk of progression from severe albuminuria to reduced GFR or ESRD [26]. However, it is unclear whether the different antidiabetic agents are all similarly effective in delaying the progression of diabetic nephropathy. This remains to be elucidated.
2.3 Other established risk factors for diabetic nephropathy
Patients with a longer duration of diabetes have a higher risk for developing nephropathy [33, 37]. Elevated blood pressure is another important independent risk factor for nephropathy [28, 33]. In the DCCT/EDIC study, lower blood pressure was associated with reduced risk for progression from moderate albuminuria to severe albuminuria or ESRD [16]. Moreover, in patients with T2D, lower blood pressure was associated with regression from moderate albuminuria to normoalbuminuria [38]. Inhibitors of the renin-angiotensin system appear to delay the progression of diabetic nephropathy more than other classes of antihypertensive agents, while the reduction in blood pressure is similar.
Dyslipidemia also appears to play a role in the pathogenesis of diabetic nephropathy. In the DCCT/EDIC study, lower low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels were associated with reduced risk for progression from moderate albuminuria to severe albuminuria or ESRD [16]. In patients with T2D, elevated total cholesterol (TC) levels are also associated with increased risk for the development of both moderately and severely increased UAE [24]. In addition, low levels of TC and TG are associated with regression from moderate albuminuria to normoalbuminuria in T2D [29]. The interventional studies with statins mentioned above also provide evidence for a role of dyslipidemia as a risk factor for diabetic nephropathy by showing that lowering LDL-C levels is associated with delayed progression of the disease.
Obesity is also associated with increased risk of diabetic nephropathy [33, 37, 39]. In DCCT, abdominal obesity, evaluated by waist circumference, was associated with a higher incidence of albuminuria, but did not predict a decline in GFR [34]. On the other hand, weight loss reduces urinary albumin excretion and prevents the decline in GFR [40, 41].
Smoking is associated with increased albuminuria and with a decline in GFR in both patients with T1D and T2D [33, 34, 42].
Advanced age increases the risk for nephropathy in both T1D and T2D [24, 33, 34]. This association appears to be independent of diabetes duration [24, 33].
In the DCCT/EDIC study, female sex was associated with reduced risk for progression from moderate albuminuria to severe albuminuria or ESRD [16]. Other studies in patients with T1D and T2D reported similar findings [24, 28].
In patients with T1D, retinopathy almost invariably precedes the development of nephropathy [43, 44]. In the DCCT/EDIC study, the absence of retinopathy was associated with reduced risk for progression from moderate albuminuria to severe albuminuria or ESRD [16]. In contrast, in T2D, almost 50% of patients with nephropathy do not have retinopathy [31, 45, 46]. However, if nephropathy is due to diabetes, retinopathy is almost always present [31, 45, 46]. Moreover, retinopathy is associated with an increased incidence of albuminuria in patients with T2D [24].
2.4 New risk factors for diabetic nephropathy
Both oxidative stress and subclinical inflammation appear to contribute to the pathogenesis of diabetic nephropathy. Elevated urinary levels of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG), a marker of oxidative stress, predict the progression of diabetic nephropathy in patients with T2D [47]. Patients with T1D or T2D and with higher levels of proinflammatory cytokines and chemokines (interleukin 6, interleukin 18, and monocyte chemoattractant protein-1), high-sensitivity C-reactive protein (hsCRP, a marker of subclinical inflammation), or adhesion molecules (soluble vascular cellular adhesion molecule-1 and soluble E-selectin) are at higher risk for developing nephropathy and for advancing to more severe kidney disease [48-51]. In patients with T1D or T2D, elevated levels of tumor necrosis factor-α receptors are also independently associated with increased incidence of impaired kidney function [52, 53]. Unfortunately, studies that evaluated the effects of antioxidant or anti-inflammatory agents for delaying the progression of diabetic nephropathy yielded disappointing results, both regarding effectiveness and safety.
The development of diabetic nephropathy is also associated with the appearance of genetic factors. Patients with T1D, and those with T2D, who have a relative with diabetic nephropathy are at increased risk for developing nephropathy [54-56]. Several studies evaluated the association between angiotensin-converting enzyme (ACE) gene polymorphisms and the risk of diabetic nephropathy, but yielded conflicting results [57-59]. Other reports suggested that polymorphisms of angiotensin-2 receptor, aldose reductase, and protein kinase C might also modulate the development of diabetic nephropathy, but these associations have to be confirmed in larger studies and different ethnicities [60-62]. More recently, genome-wide association studies (GWAS) identified several loci associated with an increased risk for diabetic nephropathy in both T1D and T2D [63-65]. African-Americans and Pima Indians have higher incidence of diabetic nephropathy than Caucasians [66, 67]. Genetic and socioeconomic factors as well as differences in glycemic and blood pressure control may contribute to these differences [66, 67].
In patients with T1D, glomerular hyperfiltration is associated with an increased risk for the development of diabetic nephropathy [68, 69]. This association is more pronounced when GFR is >125 ml/min [68]. Notably, almost half newly-diagnosed patients with T1D have glomerular hyperfiltration [70].
3. Diagnosis of diabetic nephropathy
Given that increased UAE is the most common feature of diabetic nephropathy, measuring albuminuria is an essential component in the diagnosis of diabetic nephropathy. According to the most recent guidelines issued by the American Diabetes Association (ADA), urinary albumin should be measured at least yearly in patients with T1D duration ≥5 years and in all patients with T2D [71]. UAE should be evaluated by measuring the urinary albumin/creatinine ratio in a spot urine sample, since it is as accurate as but more convenient than the measurement of UAE in a 24-h urine collection [71]. UAE varies over time, and therefore 2 or more urine specimens collected within a period of 3-6 months should show increased albumin excretion before making a diagnosis of nephropathy [71]. Moreover, urinary tract infections or other febrile infections, recent exercise, heart failure, and uncontrolled hyperglycemia or hypertension also increase UAE [71]. Therefore, UAE should not be measured in patients with febrile infections. The patients should avoid exercise before testing, and glucose levels and blood pressure should be controlled before evaluating UAE.
A substantial proportion of patients with T1D or T2D has decreased GFR, despite normal urinary albumin [29-32]. Therefore, creatinine levels should be measured, and GFR should be calculated at least annually in all patients with diabetes regardless of the presence of increased UAE [71]. Several equations have been developed for estimating GFR. The Modified Diet in Renal Disease (MDRD) Study equation underestimates GFR, particularly in patients with GFR >90 ml/min/1.73m2 [72]. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation appears to be more accurate than the MDRD equation in estimating GFR, particularly at higher GFR [73-75]. Accordingly, the ADA recommends the use of the CKD-EPI over the MDRD equation for estimating GFR in patients with T1D or T2D [71].
Cystatin C is a 13.3 kDa protein that is freely filtered in the glomeruli before it is reabsorbed and catabolized in the renal tubular cells [76]. Its serum levels are independent of muscle mass [76]. Cystatin-C levels are a better marker of renal function than serum creatinine levels or the Cockcroft-Gault equation [77]. Changes in cystatin-C over time strongly correlate with changes in GFR [78]. However, cystatin-C-based equations for the calculation of GFR are more accurate than the MDRD or CKD-EPI equations [79, 80]. However, studies in this topic are few. More data are needed to clarify whether cystatin-C is more sensitive and specific for evaluating renal function in diabetic patients than creatinine-based equations.
4. Novel markers for the early diagnosis of diabetic nephropathy
In patients with T2D, smaller studies have suggested that elevated urinary transferrin excretion rates predict the development of moderate albuminuria [81, 82]. Markers of tubular damage, including urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule 1 (KIM-1), liver-type fatty acid binding protein, and glycoprotein non-metastatic melanoma B, predict a decrease in GFR in both T1D and T2D more rapidly, but have no incremental predictive value over other established risk factors for the progression of diabetic nephropathy, particularly UAE [83-86]. On the other hand, a small study in patients with T1D suggested that low urinary levels of KIM-1 and N-acetyl-β-D-glucosaminidase (NAG) are independently associated with regression from moderate albuminuria to normoalbuminuria [87]. A nested case-control study in DCCT also reported that elevated urinary NAG levels independently predict the development of moderate and severe albuminuria [88].
Urinary proteome analysis also appears to be a promising new modality for the early diagnosis of diabetic nephropathy. It detects a variety of non-albumin urinary proteins. Small studies in patients with T1D or T2D reported that a variety of proteins detected with this method, including collagen fragments and tubular proteins, independently predicts the progression to moderate or severe albuminuria or GFR decline [89-91]. However, the high cost and limited availability of this method are important barriers for its wider implementation.
5. Conclusions
A variety of risk factors promotes the development and progression of diabetic nephropathy, including high glucose levels, obesity, dislipidemia, elevated blood pressure, oxidative stress, and others. Most of these risk factors are modifiable. Therefore, their intensive management is essential for preventing and delaying the decline in renal function. Many of these risk factors are also associated with a higher incidence of cardiovascular events, further supporting the importance of their management.
New (genetic) markers for diabetic nephropathy are being investigated. Their determination may contribute to an early and improved treatment and probably to an early identification of individuals at risk of developing the disease. The identification of novel mechanisms involved in the pathogenesis of diabetic nephropathy may also result in the development of new treatments for both micro- and macrovascular complications of diabetes. These advancements are subject of present and future research.
Early diagnosis of diabetic nephropathy is a pivotal component in the management of patients with both T1D and T2D. Glomerular filtration rate and UAE are easy to perform and inexpensive, but are considerably underused. This is a drawback in clinical practice, highlighting a major gap between necessity and reality in the management of diabetic nephropathy. Measurement of cystatin-C levels is more specific than creatinine for evaluating renal function, but it is more costly, and limited availability are substantial barriers to its wider implementation.
The existence of new markers may allow the earlier diagnosis of this frequent and high-risk complication, but further studies are needed to clarify their additive predictive value, and to define their cost-benefit ratio.
Disclosures: The authors report no conflict of interests.
References
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014. 103:137-149. [DOD] [CrossRef]
- de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011. 305:2532-2539. [DOD] [CrossRef]
- United States Renal Data System, 2014 USRDS annual data report: An overview of the epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2014. [DOD]
- Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR, Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012. 380:807-814. [DOD] [CrossRef]
- Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010. 375:2073-2081. [DOD] [CrossRef]
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001. 345:851-860. [DOD] [CrossRef]
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001. 345:861-869. [DOD] [CrossRef]
- Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001. 345:870-878. [DOD] [CrossRef]
- Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J, Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004. 351:1952-1961. [DOD] [CrossRef]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998. 352:854-865. [DOD] [CrossRef]
- Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK, Treating to New Targets Investigators. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: the TNT (Treating to New Targets) study. J Am Coll Cardiol 2008. 51:1448-1454. [DOD] [CrossRef]
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010. 55:1266-1273. [DOD] [CrossRef]
- Athyros VG, Mikhailidis DP, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, Elisaf M. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004. 57:728-734. [DOD] [CrossRef]
- Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK, Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007. 2:1131-1139. [DOD] [CrossRef]
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993. 329:977-986. [DOD] [CrossRef]
- de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD, White NH, Danis RP, Davis MD, Hainsworth D, Hubbard LD, Nathan DM, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011. 171:412-420. [DOD] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998. 352:837-853. [DOD] [CrossRef]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998. 352:854-865. [DOD] [CrossRef]
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008. 358:2560-2572. [DOD] [CrossRef]
- Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010. 376:419-430. [DOD] [CrossRef]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009. 360:129-139. [DOD] [CrossRef]
- Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 1984. 311:89-93. [DOD] [CrossRef]
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982. 1:1430-1432. [DOD] [CrossRef]
- Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ 1997. 314(7083):783-788. [DOD] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013. 113:S1-S150. [DOD]
- de Boer IH, Afkarian M, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Renal outcomes in patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 2014. 25:2342-2350. [DOD] [CrossRef]
- Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, de Boer IH, Zinman B, Lachin J, Epidemiology of Diabetes Interventions and Complications Study Group. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010. 33:1536-1543. [DOD] [CrossRef]
- Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, Binder C, Parving HH. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004. 328:1105. [DOD] [CrossRef]
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003. 348:2285-2293. [DOD] [CrossRef]
- Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int 2010. 77:57-64. [DOD] [CrossRef]
- Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003. 289:3273-3277. [DOD] [CrossRef]
- MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004. 27:195-200. [DOD] [CrossRef]
- Tapp RJ, Shaw JE, Zimmet PZ, Balkau B, Chadban SJ, Tonkin AM, Welborn TA, Atkins RC. Albuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am J Kidney Dis 2004. 44:792-798. [DOD] [CrossRef]
- de Boer IH, Sibley SD, Kestenbaum B, Sampson JN, Young B, Cleary PA, Steffes MW, Weiss NS, Brunzell JD, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol 2007. 18:235-243. [DOD] [CrossRef]
- Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med 1994. 330:15-18. [DOD] [CrossRef]
- Pavkov ME, Knowler WC, Bennett PH, Looker HC, Krakoff J, Nelson RG. Increasing incidence of proteinuria and declining incidence of end-stage renal disease in diabetic Pima Indians. Kidney Int 2006. 70:1840-1846. [DOD] [CrossRef]
- Cederholm J, Eliasson B, Nilsson PM, Weiss L, Gudbjörnsdottir S, Steering Committee of the Swedish National Diabetes Register. Microalbuminuria and risk factors in type 1 and type 2 diabetic patients. Diabetes Res Clin Pract 2005. 67:258-266. [DOD] [CrossRef]
- Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 2005. 54:2983-2987. [DOD] [CrossRef]
- Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol 2006. 17:1695-1702. [DOD] [CrossRef]
- Morales E, Valero MA, Leon M, Hernandez E, Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis 2003. 41:319-327. [DOD] [CrossRef]
- Saiki A, Nagayama D, Ohhira M, Endoh K, Ohtsuka M, Koide N, Oyama T, Miyashita Y, Shirai K. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes (Lond) 2005. 29:1115-1120. [DOD] [CrossRef]
- Gambaro G, Bax G, Fusaro M, Normanno M, Manani SM, Zanella M, Dangelo A, Fedele D, Favaro S. Cigarette smoking is a risk factor for nephropathy and its progression in type 2 diabetes mellitus. Diabetes Nutr Metab 2001. 14:337-342. [DOD]
- Parving HH, Hommel E, Mathiesen E, Skott P, Edsberg B, Bahnsen M, Lauritzen M, Hougaard P, Lauritzen E. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J (Clin Res Ed) 1988. 296:156-160. [DOD] [CrossRef]
- Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990. 39:1116-1124. [DOD] [CrossRef]
- Parving HH, Gall MA, Skott P, Jorgensen HE, Lokkegaard H, Jorgensen F, Nielsen B, Larsen S. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 1992. 41:758-762. [DOD] [CrossRef]
- Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int 2000. 58:1719-1731. [DOD] [CrossRef]
- Hinokio Y, Suzuki S, Hirai M, Suzuki C, Suzuki M, Toyota T. Urinary excretion of 8-oxo-7, 8-dihydro-2'-deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia 2002. 45:877-882. [DOD] [CrossRef]
- Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 2002. 51:1157-1165. [DOD] [CrossRef]
- Araki S, Haneda M, Koya D, Sugimoto T, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A. Predictive impact of elevated serum level of IL-18 for early renal dysfunction in type 2 diabetes: an observational follow-up study. Diabetologia 2007. 50:867-873. [DOD] [CrossRef]
- Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk CG, Tarnow L, Parving HH. Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest 2008. 68:731-738. [DOD] [CrossRef]
- Wolkow PP, Niewczas MA, Perkins B, Ficociello LH, Lipinski B, Warram JH, Krolewski AS. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol 2008. 19:789-797. [DOD] [CrossRef]
- Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, Mayadas TN, Warram JH, Krolewski AS. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012. 23:516-524. [DOD] [CrossRef]
- Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 2012. 23:507-515. [DOD] [CrossRef]
- Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 1989. 320:1161-1165. [DOD] [CrossRef]
- Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1990. 33:438-443. [DOD] [CrossRef]
- Satko SG, Langefeld CD, Daeihagh P, Bowden DW, Rich SS, Freedman BI. Nephropathy in siblings of African Americans with overt type 2 diabetic nephropathy. Am J Kidney Dis 2002. 40:489-494. [DOD] [CrossRef]
- Jeffers BW, Estacio RO, Raynolds MV, Schrier RW. Angiotensin-converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus and its relationship with diabetic nephropathy. Kidney Int 1997. 52:473-477. [DOD] [CrossRef]
- Boright AP, Paterson AD, Mirea L, Bull SB, Mowjoodi A, Scherer SW, Zinman B, DCCT/EDIC Research Group. Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes 2005. 54:1238-1244. [DOD] [CrossRef]
- Hadjadj S, Tarnow L, Forsblom C, Kazeem G, Marre M, Groop PH, Parving HH, Cambien F, Tregouet DA, Gut IG, et al. Association between angiotensin-converting enzyme gene polymorphisms and diabetic nephropathy: case-control, haplotype, and family-based study in three European populations. J Am Soc Nephrol 2007. 18:1284-1291. [DOD] [CrossRef]
- Pettersson-Fernholm K, Fröjdö S, Fagerudd J, Thomas MC, Forsblom C, Wessman M, Groop PH, FinnDiane Study Group. The AT2 gene may have a gender-specific effect on kidney function and pulse pressure in type I diabetic patients. Kidney Int 2006. 69:1880-1884. [DOD] [CrossRef]
- Shah VO, Scavini M, Nikolic J, Sun Y, Vai S, Griffith JK, Dorin RI, Stidley C, Yacoub M, Vander Jagt DL, Eaton RP, Zager PG. Z-2 microsatellite allele is linked to increased expression of the aldose reductase gene in diabetic nephropathy. J Clin Endocrinol Metab 1998. 83:2886-2891. [DOD]
- Ma RC, Tam CH, Wang Y, Luk AO, Hu C, Yang X, Lam V, Chan AW, Ho JS, Chow CC, Tong PC, Jia W, Ng MC, So WY, Chan JC. Genetic variants of the protein kinase C-beta 1 gene and development of end-stage renal disease in patients with type 2 diabetes. JAMA 2010. 304:881-889. [DOD] [CrossRef]
- Igo RP Jr, Iyengar SK, Nicholas SB, Goddard KA, Langefeld CD, Hanson RL, Duggirala R, Divers J, Abboud H, Adler SG, et al. Genomewide linkage scan for diabetic renal failure and albuminuria: the FIND study. Am J Nephrol 2011. 33:381-389. [DOD] [CrossRef]
- Thameem F, Igo RP Jr, Freedman BI, Langefeld C, Hanson RL, Schelling JR, Elston RC, Duggirala R, Nicholas SB, Goddard KA, et al. A genome-wide search for linkage of estimated glomerular filtration rate (eGFR) in the Family Investigation of Nephropathy and Diabetes (FIND). Plos One 2013. 8:e81888. [DOD] [CrossRef]
- Pezzolesi MG, Poznik GD, Mychaleckyj JC, Paterson AD, Barati MT, Klein JB, Ng DP, Placha G, Canani LH, Bochenski J, et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 2009. 58:1403-1410. [DOD] [CrossRef]
- Nelson RG, Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetic kidney disease in Pima Indians. Diabetes Care 1993. 16:335-341. [DOD] [CrossRef]
- Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA 1992. 268:3079-3084. [DOD] [CrossRef]
- Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy - an 8-year prospective study. Kidney Int 1992. 41:822-828. [DOD] [CrossRef]
- Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 2009. 52:691-697. [DOD] [CrossRef]
- Cotroneo P, Manto A, Todaro L, Manto A Jr, Pitocco D, Saponara C, Vellante C, Maussier ML, D'Errico G, Magnani P, Ghirlanda G. Hyperfiltration in patients with type I diabetes mellitus: a prevalence study. Clin Nephrol 1998. 50:214-217. [DOD]
- American Diabetes Association. Standards of medical care in diabetes 2015. Diabetes Care 2015. 38(Suppl 1):S1-S94. [DOD]
- Chudleigh RA, Dunseath G, Evans W, Harvey JN, Evans P, Ollerton R, Owens DR. How reliable is estimation of glomerular filtration rate at diagnosis of type 2 diabetes? Diabetes Care 2007. 30:300-305. [DOD]
- Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol 2011. 6:1963-1972. [DOD] [CrossRef]
- Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010. 5:1003-1009. [DOD] [CrossRef]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009. 150:604-612. [DOD] [CrossRef]
- Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: present and future. World J Diabetes 2014. 5:763-776. [DOD] [CrossRef]
- Mussap M, Dalla Vestra M, Fioretto P, Saller A, Varagnolo M, Nosadini R, Plebani M. Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int 2002. 61:1453-1461. [DOD] [CrossRef]
- Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 2005. 16:1404-1412. [DOD] [CrossRef]
- Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008. 51:395-406. [DOD] [CrossRef]
- Iliadis F, Didangelos T, Ntemka A, Makedou A, Moralidis E, Gotzamani-Psarakou A, Kouloukourgiotou T, Grekas D. Glomerular filtration rate estimation in patients with type 2 diabetes: creatinine- or cystatin C-based equations? Diabetologia 2011. 54:2987-2994. [DOD]
- Kazumi T, Hozumi T, Ishida Y, Ikeda Y, Kishi K, Hayakawa M, Yoshino G. Increased urinary transferrin excretion predicts microalbuminuria in patients with type 2 diabetes. Diabetes Care 1999. 22:1176-1180. [DOD] [CrossRef]
- Narita T, Hosoba M, Kakei M, Ito S. Increased urinary excretions of immunoglobulin g, ceruloplasmin, and transferrin predict development of microalbuminuria in patients with type 2 diabetes. Diabetes Care 2006. 29:142-144. [DOD] [CrossRef]
- Nielsen SE, Reinhard H, Zdunek D, Hess G, Gutierrez OM, Wolf M, Parving HH, Jacobsen PK, Rossing P. Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res Clin Pract 2012. 97:71-76. [DOD] [CrossRef]
- Chou KM, Lee CC, Chen CH, Sun CY. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. Plos One 2013. 8:e54863. [DOD] [CrossRef]
- Conway BR, Manoharan D, Manoharan D, Jenks S, Dear JW, McLachlan S, Strachan MW, Price JF. Measuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factors. Kidney Int 2012. 82:812-818. [DOD] [CrossRef]
- Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int 2011. 79:1113-1118. [DOD] [CrossRef]
- Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int 2011. 79:464-470. [DOD] [CrossRef]
- Kern EF, Erhard P, Sun W, Genuth S, Weiss MF. Early urinary markers of diabetic kidney disease: a nested case-control study from the Diabetes Control and Complications Trial (DCCT). Am J Kidney Dis 2010. 55:824-834. [DOD] [CrossRef]
- Zürbig P, Jerums G, Hovind P, Macisaac RJ, Mischak H, Nielsen SE, Panagiotopoulos S, Persson F, Rossing P. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes 2012. 61:3304-3313. [DOD] [CrossRef]
- Schlatzer D, Maahs DM, Chance MR, Dazard JE, Li X, Hazlett F, Rewers M, Snell-Bergeon JK. Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes Care 2012. 35:549-555. [DOD] [CrossRef]
- Rossing K, Mischak H, Dakna M, Zürbig P, Novak J, Julian BA, Good DM, Coon JJ, Tarnow L, Rossing P, PREDICTIONS Network. Urinary proteomics in diabetes and CKD. J Am Soc Nephrol 2008. 19:1283-1290. [DOD] [CrossRef]
This article has been cited by other articles:

|
Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus
Giribabu N, Karim K, Kilari EK, Salleh N
J Ethnopharmacol 2017. 205:123-137
|
|

|
The beneficial effects of zinc on diabetes-induced kidney damage in murine rodent model of type 1 diabetes mellitus
Yang F, Li B, Dong X, Cui W, Luo P
J Trace Elem Med Biol 2017. 42:1-10
|
|
.jpg)
|
Clinical efficacy and safety of tripterygium glycosides in treatment of stage IV diabetic nephropathy: A meta-analysis
Hong Y, Gui Z, Cai X, Lan L
Open Med (Wars) 2016. 11(1):611-617
|
|
|
Maternal Obesity Promotes Diabetic Nephropathy in Rodent Offspring
Glastras SJ, Tsang M, Teh R, Chen H, McGrath RT, Zaky AA, Pollock CA, Saad S
Sci Rep 2016. 6:27769
|
|
|