Chapter IV. Cardiovascular Autonomic Neuropathy
| Rev Diabet Stud,
2015,
12(1-2):213-219 |
DOI 10.1900/RDS.2015.12.213 |
Simplified Diagnosis of Cardiovascular Autonomic Neuropathy in Type 2 Diabetes Using Ewing's Battery
Kalliopi Pafili1, Grigorios Trypsianis2, Dimitrios Papazoglou1, Efstratios Maltezos1, Nikolaos Papanas1
1Diabetes Clinic, Second Department of Internal Medicine, Democritus University of Thrace, University Hospital of Alexandroupolis, Greece
2Department of Medical Statistics, Medical Faculty, Democritus University of Thrace, Alexandroupolis, Greece
Address correspondence to: Kalliopi Pafili, Diabetes Clinic, Second Department of Internal Medicine, Democritus University of Thrace, University Hospital of Alexandroupolis, Alexandroupolis 68100, Greece, email: kpafili@hotmail.com
Manuscript submitted March 12, 2015; resubmitted March 29, 2015; accepted April 10, 2015.
Keywords: diabetic retinopathy, macular edema, proliferative diabetic retinopathy, vascular endothelial growth factor
Abstract
AIM: To find a potential simplification of the established Ewing's battery for the diagnosis of cardiovascular autonomic neuropathy (CAN) in type 2 diabetes (T2D). METHODS: We included 152 patients (92 men) with mean age 64.51 ± 7.85 years and median diabetes duration of 12 years. Ewing's battery was used as the gold standard for the diagnosis of CAN. Against this, we compared the results from each test and their combinations. RESULTS: The 30:15 ratio exhibited the best diagnostic performance (AUC = 0.817, 95% CI: 0.730-0.903, p < 0.001), with 96% sensitivity, 65% specificity, and 94% negative predictive value (NPV). The corresponding values for the Valsalva ratio (VR) were 62%, 92%, and 85%, respectively. The 30:15 ratio was the strongest independent predictor of neuropathy in multivariate regression analysis; low levels yielded an odds ratio (OR) of 21.14 for CAN. The rise in diastolic blood pressure and the expiration/inspiration/VR ratio (E/I/VR) were also identified as independent predictors of CAN, with 9.45 and 10.79 ORs, respectively. CONCLUSIONS: The 30:15 ratio has the best diagnostic accuracy, primarily in the exclusion of CAN, by virtue of its very high sensitivity and NPV. If this ratio is positive for CAN, the VR, the rise in diastolic blood pressure, and the E/I/VR may be useful to increase diagnostic accuracy. This procedure is a simplified diagnostic approach that merits further evaluation to enable wider screening for CAN.
Abbreviations: AUC – area under the curve; CAN – cardiovascular autonomic neuropathy; CI – confidence interval; E/I – expiration/inspiration; E/I/VR – expiration/inspiration/Valsalva ratio; E/I/30:15 – expiration/inspiration/30:15; NPV – negative predictive value; OR – odds ratio; PPV – positive predictive value; T2D – type 2 diabetes; VR – Valsalva ratio
1. Introduction
In diabetes, cardiovascular autonomic neuropathy (CAN) refers to impaired autonomic control of the heart and vessels [1, 2]. It is considered the most important manifestation of diabetic autonomic neuropathy [3], because it is associated with life-threatening clinical entities such as silent myocardial ischemia [4], coronary artery disease [5], peri-operative cardiovascular instability [6], QT segment prolongation [7], and stroke [8], resulting in increased mortality [9, 10].
Timely diagnosis of CAN is useful to avoid complications [3, 9]. In clinical practice, the 5 non-invasive cardiovascular reflex tests proposed by Ewing and Clarke more than 30 years ago are considered the cornerstone of diagnosis. The tests are widely used as they provide good sensitivity, specificity, and reproducibility, and they are well-standardized and easily performed [8, 11, 12].
Ewing and Clarke recommended performing all 5 tests (the so-called Ewing's battery) for the diagnosis of CAN [12]. However, these tests are time-consuming and involve difficulties that prevent the performance of several tests, for example:
- Arthritis or difficulties in patient compliance may prevent performing the handgrip test.
- It is frequently impossible to carry out the Valsalva maneuver as it also depends on patient effort [12].
- Mobility challenges or the presence of Charcot osteoarthropathy frequently prevents the performance of the lying-to-standing test.
- Postural fall in blood pressure cannot be reliably assessed in patients with fluid retention [13].
- Forceful breathing is not indicated for patients with proliferative retinopathy [12].
Also, there is still debate about the diagnostic criteria and staging of CAN [8]. Therefore, the aim of this study was to describe a potential simplification of the established Ewing’s battery for the diagnosis of CAN in type 2 diabetes (T2D) by utilizing results from each test and combinations of these results.
2. Methods and patients
2.1 Patients
This study included 152 patients (92 men, 60 women) with mean age 64.51 ± 7.85 years and median diabetes duration of 12 years (inter-quartile range 8-20 years). The patients attended the Diabetes Clinic of the Second Department of Internal Medicine at Democritus University of Thrace, Greece. The study was approved by the institutional ethics committee. All patients provided their informed consent. Demographic and clinical characteristics are summarized in Table 1.
Table
1.
Patient characteristics |
|
 |
 |
Legend:
CAN – cardiovascular autonomic neuropathy, IQR – inter-quartile range, SD – standard deviation. |
|
To avoid a selection bias, we applied a random patient enrollment process. The first two patients to attend a scheduled appointment in the Diabetes Clinic every day from Monday to Friday between October 2013 and August 2014 were enrolled in the study. Appointments in our hospital are not arranged by doctors nor can doctors interfere with the order of patients' appointments. One week before each examination, a member of the administrative staff (not involved in the study) handed over an appointment list to the examiner. Each patient included in the study was given information on the study, asked for consent, and advised on what should be avoided (e.g. food and certain medications, as explained below) before the examination procedure. Only the first two patients could be included because the examination for Ewing's battery needs to be performed in the early morning [12]. The examination was consistently carried out by one examiner (KP), who was blinded to patients' symptoms and clinical history.
Exclusion criteria were as follows:
- Age >85 years old
- Mentally ill patients or those unable to complete the tests
- Arrhythmia, including atrial fibrillation
- Severe illness, such as malignancy and severe infection
- Severe hypoglycemia
- Liver cirrhosis
- Heart failure
- Alcoholism
Patients with proliferative retinopathy were excluded from the Valsalva examination.
The gold standard for the diagnosis of CAN in the study was Ewing’s battery, which includes 5 standardized tests [12]. Patients were asked to fast for 12 h before the procedure, and to avoid taking antidepressants, neuroleptics, caffeine, nicotine, or antihistamines. The computer-aided system Varia cardio TF5 (MIE Medical Research, Leeds, UK) was used for patient examination. In brief, tests of parasympathetic cardiovagal regulation included heart rate analysis in the standing position (the 30:15 ratio), heart rate variation with deep breathing, and the Valsalva ratio (VR) [3, 12, 14, 15]. Tests of sympathetic adrenergic vascular regulation included blood pressure analysis in the standing position, the Valsalva maneuver, and sustained handgrip [3, 12, 14, 15]. Heart rate variation was assessed by electrocardiogram recordings of beat-to-beat (R-R intervals) between two consecutive electrocardiogram R waves [3, 12, 14, 15]. The tests were carried out between 07:00 and 09:00 am in a quiet environment with steady temperature levels between 22-24° C [14, 15].
The expiration/inspiration (E/I) ratio was calculated as the mean of the longest R-R interval during expiration divided by the mean of the shortest R-R interval during inspiration, while the patient lay quietly and breathed deeply with an electrocardiogram that recorded heart rate variation over six breathing cycles [14, 15]. The ratio of postural change was the ratio of the longest R-R interval during beats 20-40 after standing to the shortest R-R interval during beats 5-25 after standing. For the heart rate response to the Valsalva manoeuver, the ratio of the longest R-R interval to the shortest R-R interval was checked during forced exhalation into the mouthpiece of a manometer against 40 mmHg for 15 seconds [14, 15]. During the two subsequent blood pressure tests, blood pressure in response to standing from a lying position and blood pressure variation before and during a sustained handgrip was recorded [3, 14, 15].
The following test results were considered as normal:
- E/I ratio above the age-related reference value [16]
- VR ≥ 1.21
- Posture ratio ≥ 1.04
- Systolic blood pressure reduction in response to standing ≤ 10 mmHg
- Diastolic blood pressure increase in response to sustained handgrip ≥ 16 mmHg [16, 17]
The following test results were considered as abnormal:
- E/I ratio below the age-related values
- VR ≤ 1.10
- Posture ratio ≤ 1.00
- Systolic blood pressure fall in response to standing ≥ 30
- Diastolic blood pressure rise in response to sustained handgrip ≤ 10 mmHg [16, 17]
Each of the items was scored as 0 for normal, 1 for borderline, and 2 for abnormal. CAN was defined as ≥2 abnormal tests [12, 16, 17].
2.2 Statistical analysis
The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM). The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to quantify the diagnostic performance of tests. Sensitivity, specificity, positive and negative predictive values were calculated, while Cohen's kappa was used to assess agreement. Student's t-test and the Mann Whitney U-test test were carried out to evaluate the potential associations between the presence or absence of neuropathy and the new indices. Multivariate logistic regression analysis including gender, age, and diabetes duration was employed to determine the ratios that could be independent predictors of neuropathy. Odds ratios (OR) and 95% confidence intervals (CI) were estimated as measures of association between the ratios of neurophysiological parameters and neuropathy. All tests were two-tailed, and significance was defined at the 5% level (p < 0.05).
3. Results
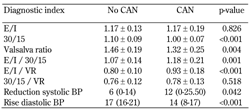
Overall, 48% (73 of 152 patients) had CAN. The 30:15 (p < 0.001) and VR ratio (p = 0.004) were significantly lower among patients with CAN, while the E/I ratio did not differ significantly between the two groups (p = 0.826). Patients with CAN exhibited a significantly greater reduction in systolic blood pressure and a smaller rise in diastolic blood pressure (p = 0.042 and p < 0.001, respectively), as compared with those who did not have CAN. The values of all indices and their ratios in relation to the presence of CAN are presented in Table 2.
Table
2.
Diagnostic indices in patients with and without CAN |
|
 |
 |
Legend:
Data are mean ± SD or median and inter-quartile range. Abbreviations: BP – blood pressure, CAN - cardiovascular autonomic neuropathy, E/I – expiration/inspiration index, IQR – inter-quartile range, SD – standard deviation, VR – Valsalva ratio. |
|
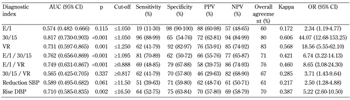
Table 3 shows the AUC for the above-mentioned markers. The 30:15 ratio showed the best diagnostic performance (AUC = 0.817, 95% CI: 0.730-0.903, p < 0.001). The following four additional indices had an AUC greater than 0.7, which indicates a high diagnostic performance:
- VR (AUC = 0.731, 95% CI: 0.597-0.865, p = 0.001)
- Rise in diastolic blood pressure (AUC = 0.710, 95% CI: 0.585-0.835, p = 0.002)
- E/I/30:15 ratio (AUC = 0.762, 95% CI: 0.656-0.869, p < 0.001)
- E/I/VR ratio (AUC = 0.749, 95% CI: 0.631-0.867, p < 0.001)
Inferior, non-significant results were obtained for the E/I ratio (p = 0.115), the 30:15/VR ratio (p = 0.337), and the reduction in systolic blood pressure (p = 0.061).
Table
3.
Performance of diagnostic indices |
|
 |
 |
Legend:
AUC – area under the curve, CAN – cardiovascular autonomic neuropathy, CI – confidence interval, DBP – diastolic blood pressure, E/I – expiration/inspiration index, NPV – negative predictive value, OR – odds ratio, PPV – positive predictive value, SBP – systolic blood pressure, VR – Valsalva ratio. |
|
Clinically important cut-off points for all these diagnostic indices were also determined by ROC curve analysis (Table 3). In particular, the optimal cut-off point of 1.050 for the 30:15 ratio yielded a sensitivity of 96% (95% CI: 88-99%), a substantial specificity of 65% (95% CI: 54-76%), with a positive predictive value (PPV) of 72% and a negative predictive value (NPV) of 94%. The cut-offs for the other four markers yielded substantial sensitivities (62% to 81%) and specificities (62% to 92%).
The overall agreement of the classification of patients according to the 5 indices with the gold standard of CAN diagnosis was over 70% (70-83%), while Cohen's kappa coefficient indicated good agreement for the 30:15 ratio (kappa = 0.606, p < 0.001), and substantial agreement for the VR, the rise in diastolic blood pressure, the E/I/30:15 ratio, and the E/I/VR ratio, with kappa values ranging from 0.387 to 0.568 (all p < 0.001). Slightly weaker results were obtained for the other three ratios (Table 3).
Furthermore, logistic regression analysis revealed higher ORs for the 30:15 ratio (OR = 44.07, 95%CI: 12.68-153.52) and the VR (OR = 18.56, 95%CI: 5.55-62.10).
Finally, in multivariate logistic regression analysis, the 30:15 ratio, the rise in diastolic blood pressure, and the E/I/VR ratio remained independent predictors of CAN. Low values for the 30:15 ratio, low values for the rise in diastolic blood pressure, and high values for the E/I/VR ratio yielded adjusted ORs for CAN of 21.14 (95% CI: 3.42-129.81, p < 0.001), 9.45 (95% CI: 2.61-34.17, p = 0.001), and 10.79 (95% CI: 2.52-46.32, p = 0.001), respectively.
4. Discussion
In an attempt to find a potential simplification of the established Ewings' battery for the diagnosis of CAN in T2D, this study has demonstrated the best AUC for the 30:15 ratio, and a good performance for 4 additional indices, including the VR, the rise in diastolic blood pressure, the E/I/30:15 ratio, and the E/I/VR ratio.
Of these indices, the 30:15 ratio emerged as the most useful diagnostic index. The 30:15 ratio also exhibited a good agreement with the gold standard of CAN diagnosis. Substantial agreement was also found for the VR, the rise in diastolic blood pressure, the E/I/30:15 ratio, and the E/I/VR ratio. Moreover, the 30:15 ratio exhibited the highest OR for CAN (OR: 44.07), followed by the VR (OR: 18.56). More importantly, a low 30:15 ratio remained the strongest independent predictor of CAN in multivariate regression analysis, followed by high levels of the E/I/VR ratio and an increase in diastolic blood pressure.
These novel findings suggest that the 30:15 ratio was the most useful index for the diagnosis of CAN. It yielded a high sensitivity, a moderately high specificity, and it was a strong predictor of CAN. The VR, the rise in diastolic blood pressure, and the E/I/VR ratio followed in diagnostic usefulness.
There have been previous attempts to simplify the diagnosis of CAN [16, 18-20]. Ewing et al. found that the 30:15 ratio could be used as a simplified test to differentiate normal subjects and diabetic patients (diabetes type not mentioned) without CAN from those with CAN [18]. However, these observations were restricted to a very small number of patients [18]. Ten years later, it was shown that the identification of CAN by the use of the Ewing tests in diabetic patients (diabetes type not mentioned) could be simplified without loss of predictive power by reducing the number of repetitions for 3 of the 5 tests, namely for Valsalva manoeuver, deep breathing, and isometric handgrip [19].
Kempler et al. proposed the beat-to-beat variation and the 30:15 ratio as the optimal initial screening tools in both diabetes types: in case of normal results, no further testing was needed [20]. More recently, Stranieri et al. proposed the deep breathing heart rate variation test as the best single screening test for the diagnosis of CAN, but suggested that additional accuracy was obtained by adding any number of the remaining tests after determining the optimal sequence of tests for each individual (diabetes type not mentioned) [16]. Our study differs from these studies in terms of design. Our study did not aim to condense the examination procedure or to establish a different sequence for the standard procedure, but to define a combination of tests showing the best accuracy for the diagnosis of CAN in T2D.
The present work has some limitations. Firstly, we did not correlate clinical findings with biochemical data. Moreover, we did not distinguish between moderate and severe CAN. However, our aim was not to identify an index of CAN severity enabling its staging, but to simplify the examination procedure for CAN diagnosis. An additional limitation is the tertiary care setting, which accounts for the very high prevalence of CAN in our series. Indeed, the patients included were predominantly middle-aged or elderly and had long diabetes duration. Therefore, it is appropriate to be cautious before applying these results to the general diabetic population, especially to younger patients, those from primary care, or those with shorter diabetes duration. Clearly, larger studies with more varied population features are needed to clarify this issue.
Another limitation is that the E/I ratio did not differ significantly between patients with and without CAN. This ratio yielded the highest specificity and PPV, but its sensitivity was the lowest, in contrast to earlier reports [15, 21]. This finding may be attributed to the need for patient compliance during the procedure. An additional issue that deserves attention is that the last Toronto consensus expert panel did not suggest applying the handgrip as part of the gold standard for clinical CAN testing [22], a recommendation which is in accordance with results of the present study. A final limitation of the present study is the lack of age-related cut-off values for the heart rate variability ratios, except in the case of the E/I ratio. However, this was an intended simplification. Age-related cut-off values were proposed in 1982 by Smith [15] and in 1992 by Ziegler et al. [23], and have been strongly recommended by the recent Toronto consensus expert panel [22]. In a series of 174 healthy subjects and 134 diabetic patients, Smith showed that the deep breathing test appreciably declines with age [15]. In 120 healthy subjects aged 15-67 years, Ziegler et al. found that age-related normal ranges for heart rate variability ratios are useful [23]. However, previous researchers similarly attempting to simplify the diagnostic procedure for CAN diagnosis did not use age-related values [16, 19].
The practical implications of the present study may be outlined as follows. The 30:15 ratio can be used as the first examination to differentiate T2D patients with and without CAN. Since its sensitivity and NPV are very high, it seems reasonable that it could serve as the best index to be applied for the exclusion of CAN. If CAN cannot be excluded by the 30:15 ratio, because its specificity is low, a combination of tests should be used to confirm or finally exclude the presence of the disease. The combination should include the VR, the rise in diastolic blood pressure, and the E/I/VR ratio. This procedure would increase diagnostic accuracy, and reduce the examination time in comparison with the full Ewing's battery.
Of note, our results also indicate that there may be a small number of CAN-positive patients who exhibit a normal 30:15 ratio. We propose that medical history, in particular a history of poor glycemic control, the presence of at least one major cardiovascular risk factor, or the presence of macro- and/or microvascular complications, points to suspected CAN in such patients. Furthermore, specific clinical findings should sensitize physicians to the presence of CAN. These may include orthostatic symptoms, unexplained tachycardia, and QT segment prolongation. Regular testing through a simplified procedure, as proposed in this study, for example at a 1-year interval in the absence of symptoms, should further enhance early CAN detection and timely intervention.
Clearly, special equipment and experienced personnel are still required to perform the examination even with the simplified approach reported here. Nonetheless, a considerable reduction in the time needed for the examination is feasible. Indeed, it takes about 5 minutes to perform the 30:15 ratio, while 20 minutes are required to conduct the 4 above-mentioned tests, and the entire examination for the 5 Ewing tests takes approximately 25 minutes. Even though a formal cost-effectiveness analysis was beyond the scope of the present work, it is conceivable that this substantial minimization of examination time may enable more widespread screening for CAN. Interestingly, the present attempt at shortening the examination time is in the same context as the suggested simplification of nerve conduction study by the sural sensory/radial motor amplitude ratio [24] and some innovations in corneal confocal microscopy [25-27]. Nonetheless, it should by no means be suggested that a simple index like the 30:15 ratio, or a combination of the 30:15 ratio with the VR, the E/I/VR, and the rise in diastolic blood pressure, could currently replace the validated 5 Ewing tests. Nor is there any evidence that the simplification of the procedure could quantify the severity of autonomic damage, as has been shown for the Ewing battery test [12].
5. Conclusions
This study has shown that the 30:15 ratio as a single examination provided the best diagnostic performance for CAN in T2D. In practice, it appears primarily useful in the exclusion of CAN by virtue of its very high sensitivity and NPV. Additional accuracy can be obtained by concurrent use of the VR, the rise in diastolic blood pressure, and the E/I/VR ratio. Our results encourage further evaluation of this simplified diagnostic approach to enable wider screening for CAN. Indeed, this complication still needs to be diagnosed earlier in more patients [22, 28] to reduce their cardiovascular morbidity and mortality [29-31].
Disclosures: The authors reported no conflicts to disclose.
References
- Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 2004. 27(12):2942-2947. [DOD] [CrossRef]
- Valensi P, Paries J, Attali JR. French Group for Research and Study of Diabetic Neuropathy Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications–the French multicenter study. Metabolism 2003. 52(7):815-820. [DOD] [CrossRef]
- Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003. 26(5):1553-1579. [DOD] [CrossRef]
- Murray D, O'Brain T, Mulrooney R, O'Suffivan DJ. Autonomic dysfunction and silent myocardial ischaemia on exercise testing in diabetes mellitus. Diabet Med 1990. 7(7):580-584. [DOD] [CrossRef]
- Kahn JK, Zola B, Juni JE, Vinik AI. Radionuclide assessment of left ventricular diastolic filling in diabetes mellitus with and without cardiac autonomic neuropathy. J Am Coll Cardiol 1986. 7(6):1303-1309. [DOD] [CrossRef]
- Burgos LG, Ebert TJ, Asiddao C, Turner LA, Pattison CZ, Wang-Cheng R, Kampine JP. Increased intraoperative cardiovascular morbidity in diabetics with autonomic neuropathy. Anesthesiology 1989. 70(4):591-597. [DOD] [CrossRef]
- Desouza CV, Bolli GB, Fonseca V. Hypoglycaemia, diabetes and cardiovascular events. Diabetes Care 2010. 33(6):1389-1394. [DOD] [CrossRef]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010. 33(10):2285-2293. [DOD] [CrossRef]
- Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007. 115(3):387-397. [DOD] [CrossRef]
- Genovely H, Pfeifer MA. RR-variation: the autonomic test of choice in diabetes. Diabetes Metab Rev 1988. 4(3):255-271. [DOD] [CrossRef]
- American Diabetes Association, American Academy of Neurology. Consensus statement. Report and recommendations of the Saint Antonio Conference on diabetic neuropathy. Diabetes 1998. 37:1000-1004. [DOD]
- Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985. 8(5):491-498. [DOD] [CrossRef]
- Campbell IW, Ewing DJ, Clarke BF. Therapeutic experience with fludrocortisones in diabetic postural hypotension. Br Med J 1976. 1(6014):872-874. [DOD] [CrossRef]
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005. 28(4):956-962. [DOD] [CrossRef]
- Smith SA. Reduced sinus arrhythmia in diabetic autonomic neuropathy: diagnostic value of an age-related normal range. Br Med J (Clin Res Ed) 1982. 285(6355):1599-1601. [DOD] [CrossRef]
- Stranieri A, Abawajy J, Kelarev A, Huda S, Chowdhury M, Jelinek HF. An approach for Ewing test selection to support the clinical assessment of cardiac autonomic neuropathy. Artif Intell Med 2013. 58(3):185-193. [DOD] [CrossRef]
- Liatis S, Marinou K, Tentolouris N, Pagoni S, Katsilambros N. Usefulness of a new indicator test for the diagnosis of peripheral and autonomic neuropathy in patients with diabetes mellitus. Diabet Med 2007. 24(12):1375-1380. [DOD] [CrossRef]
- Ewing DJ, Campbell IW, Murray A, Neilson JM, Clarke BF. Immediate heart-rate response to standing: simple test for autonomic neuropathy in diabetes. Br Med J 1978. 1(6106):145-147. [DOD] [CrossRef]
- Mustonen J, Länsimies E, Uusitupa M, Talwar S, Hyödynmaa S, Kärkkäinen A. Testing of autonomic cardiovascular regulation - methodological considerations. Clin Physiol 1989. 9(3):249-257. [DOD] [CrossRef]
- Kempler P, Varadi A, Tamas G. Which battery of cardiovascular autonomic function tests - suggestion for a rational diagnostic model. Diabetologia 1990. 33(10):640. [DOD] [CrossRef]
- Löllgen D, Müeck-Weymann M, Beise RD. The deep breathing test: median-based expiration-inspiration difference is the measure of choice. Muscle Nerve 2009. 39(4):536-544. [DOD] [CrossRef]
- Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011. 27(7):639-653. [DOD] [CrossRef]
- Ziegler D, Laux G, Dannehl K, Spüler M, Mühlen H, Mayer P, Gries FA. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med 1992. 9(2):166-175. [DOD] [CrossRef]
- Papanas N, Trypsianis G, Giassakis G, Vadikolias K, Christakidis D, Piperidou H, Efstratiadis G, Maltezos E. The sural sensory/radial motor amplitude ratio for the diagnosis of peripheral neuropathy in type 2 diabetic patients. Hippokratia 2010. 14(3):198-202. [DOD]
- Bucher F, Schneider C, Blau T, Cursiefen C, Fink GR, Lehmann HC, Heindl LM. Small-fiber neuropathy is associated with corneal nerve and dendritic cell alterations: an in vivo confocal microscopy study. Cornea 2015. 34(9):1114-1119. [DOD] [CrossRef]
- Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Brüggemann J, Strom A, Peschel S, Köhler B, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014. 63(7):2454-2463. [DOD] [CrossRef]
- Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep 2013. 13(4):488-499. [DOD] [CrossRef]
- Körei AE, Istenes I, Papanas N, Kempler P. Small-Fiber Neuropathy: A Diabetic Microvascular Complication of Special Clinical, Diagnostic, and Prognostic Importance. Angiology 2015. In press. [DOD]
- Vinik AI, Maser RE, Ziegler D. Neuropathy: the crystal ball for cardiovascular disease? Diabetes Care 2010. 33(7):1688-1690. [DOD]
- Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med 1993. 10(9):820-824. [DOD] [CrossRef]
- Putz Z, Nemeth N, Istenes I, Martos T, Gandhi RA, Körei AE, Hermanyi Z, Szathmari M, Jermendy G, Tesfaye S, et al. Autonomic dysfunction and circadian blood pressure variations in people with impaired glucose tolerance. Diabet Med 2013. 30(3):358-362. [DOD] [CrossRef]
|