Review
| Rev Diabet Stud,
2017,
14(2-3):260-268 |
DOI 10.1900/RDS.2017.14.260 |
The Effects of Vitamin D Supplementation in Newly Diagnosed Type 1 Diabetes Patients: Systematic Review of Randomized Controlled Trials
Elina Gregoriou1, Ioannis Mamais2, Irene Tzanetakou3, Giagkos Lavranos4, Stavri Chrysostomou5
1Department of Life Sciences, European University Cyprus, Engomi, 1516 Nicosia-Cyprus
2Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Greece
3Department of Life Sciences, European University of Cyprus, Nicosia, Cyprus, Engomi, 1516 Nicosia-Cyprus
4Department of Health Sciences, European University Cyprus, Engomi, 1516 Nicosia-Cyprus
5Department of Life Sciences, School of Science, European University Cyprus, Engomi, 1516 Nicosia-Cyprus
Address correspondence to: Stavri Chrysostomou, Department of Life Sciences, School of Science, European University Cyprus, Diogenis Str. 6, Engomi, P.O. Box 22006, 1516 Nicosia-Cyprus, e-mail: s.chrysostomou@euc.ac.cy
Manuscript submitted October 17, 2016; resubmitted May 22, 2017; accepted May 23, 2017.
Keywords: type 1 diabetes, new-onset diabetes, vitamin D, randomized control trial, insulin, HbA1c, pancreatic beta-cell, stimulated C-peptide, fasting C-peptide
Abstract
AIM: The aim of this study was to examine the effects of vitamin D supplementation in patients newly diagnosed with type 1 diabetes (T1D) assessed by insulin needs and changes in glycemic indices, as evidenced by randomized controlled trials (RCTs). METHODS: A total of 7 RCTs were retrieved from PubMed/Medline and EBSCO databases by MeSH term search, and were reviewed systematically. The RCTs included examined the effects of alphacalcidole (n = 2), cholecalciferol (n = 2), and calcitriol (n = 3) supplementation on changes in daily insulin dose (DID), fasting Cpeptide (FCP), stimulated C-peptide (SCP), and HbA1c. In total, 287 individuals, diagnosed with T1D within a period of 4 weeks to 1 year and aged between 5 to 38 years, were examined. RESULTS: Significant positive effects on DID, FCP, and SCP levels were observed after supplementation with alphacalcidole and cholecalciferol, whereas supplementation with calcitriol showed no effect. CONCLUSIONS: Vitamin D supplementation in the form of alphacalcidole and cholecalciferol appears to be beneficial in the treatment of T1D patients by attenuating the natural history of the disease.
Abbreviations: 1,25(OH)2D - 1,25-dihydroxyvitamin D; 1,25(OH)2D3 - 1,25-dihydroxyvitamin D3; 1α-OHase - 25-hydroxyvitamin D3-1α-hydroxylase; 25(OH)D - 25-hydroxyvitamin D; CCBRG - Cochrane Collaboration Back Review Group; DID - daily insulin dose; FCP - fasting C-peptide; FNB - Food and Nutrition Board; HbA1c - hemoglobin A1c; HLA - human leucocyte antigen; LADA - latent autoimmune diabetes of the adult; mRNA - messenger RNA; PRISMA - Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines; PICOS - Participants, Interventions, Comparisons, Outcomes, and Study design approaches; RCT - randomized controlled trial; RDA - recommended dietary allowance; SCP - stimulated C-peptide; SMBG - self-monitored blood glucose; T1D - type 1 diabetes; T2D - type 2 diabetes; VDR - vitamin D receptor
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease leading to β-cell destruction in the pancreas and concurrent insulin deficiency [1, 2]. Data from large epidemiologic studies indicate that the incidence of T1D has been increasing by 2-5% worldwide, and that the prevalence of T1D is approximately 1 in 300 US citizens over the age of 18 [3]. This alarming trend has triggered extensive research to detect the mechanisms involved in T1D pathophysiology and develop new agents. This research has led to the discovery of associations with multiple endocrine, paracrine, and immunological pathways, including those related to vitamin D [4].
Vitamin D, also known as calciferol, exists in two major forms, namely vitamin D2 or ergocalciferol and vitamin D3 or cholecalciferol, which are mainly found in plant and animal products, respectively [4, 5]. Cholecalciferol can also be synthesized in the skin through ultraviolet B radiation [4]. The inactive forms of vitamin D are transported to the liver where they are hydroxylated and converted into 25-hydroxyvitamin D (25(OH)D) or calcidiol. Thereafter, calcidiol is converted to the biologically active form of the vitamin, 1,25-dihydroxyvitamin D (1,25(OH)2D) or calcitriol [6].
Alphacalcidole is another form of vitamin D3, which is used extensively in the treatment of renal osteopathy as it allows the ingestion of higher doses of vitamin D with a reduced risk of hypercalcemia, and it can be produced at relatively low costs compared to calcitriol [7]. Previous studies have demonstrated that the daily use of alphacalcidole is well tolerated with few observed adverse effects [8, 9]. Nevertheless, the recommended dietary allowance (RDA) published by the Food and Nutrition Board (FNB) at the Institute of Medicine of The National Academies (formerly National Academy of Sciences) for people between 1 and 70 years, is no higher than 600 IU/day, while for the elderly (>70 years) it is 800 IU/day [10].
In vitro studies in human cell lines and animal studies indicate that vitamin D exhibits anti-inflammatory and immunomodulatory properties [11-13]. Based on the findings of preclinical studies, vitamin D seems to play a regulatory role in insulin secretion, β-cell survival, and calcium flux within pancreatic β-cells [6]. Furthermore, numerous studies have shown that vitamin D supplementation may restore glucose-stimulated insulin [4, 14].
The potential biological mechanisms by which the insufficient levels of vitamin D might be associated with the development of T1D remain unclear. Primary evidence from in vitro studies has shown that pancreatic tissue, specifically pancreatic islets, express vitamin D receptors (VDRs) and 25-hydroxyvitamin D3-1α-hydroxylase (1α-OHase) [15], an enzyme that catalyzes the hydroxylation of 25(OH)D (calcifediol) to 1,25(OH)2D3 (calcitriol). Furthermore, the pancreas responds to circulating levels of 1,25(OH)2D3 [13]. Thus, the expression and activity of 1α-OHase in pancreatic islets and the timely response of insulin-secreting cells to 1,25(OH)2D3 suggest that locally produced 1,25(OH)2D3 is vital for physiological islet function [15].
There is evidence supporting the hypothesis of a 1,25(OH)2D3-induced activation of insulin biosynthesis. However, results remain divergent. While an in vitro effect was found, including the activation of pre-proinsulin messenger RNA (mRNA) in 48-h cultured islets from fed rats, no effect of 1,25(OH)2D3 on insulin biosynthesis was observed in vivo [16]. Furthermore, supplementation of vitamin D-deficient animals with vitamin D restored insulin secretion [16].
Vitamin D may act synergistically or additively with other nutrients on insulin secretion, e.g. with calcium [15]. However, our understanding of the exact mechanism by which vitamin D and calcium or other nutrient-to-nutrient interactions may enhance β-cell function or improve insulin resistance remains limited.
Despite a large amount of evidence from experimental and observational studies supporting the promising outcomes of the effects of vitamin D on glucose metabolism and the immune system, results from clinical studies remain inconsistent, which makes it impossible to recommend vitamin D supplementation for the treatment of T1D [1]. Thus, the aim of this systematic review was to examine the effects of vitamin D supplementation on insulin deficiency in newly diagnosed T1D patients, as reported by randomized controlled trials (RCTs). A secondary aim was to review information on the most effective, safe, and appropriate type of vitamin D, as well as its dosage and duration of supplementation required for the potential treatment of T1D.
2. Methodology
2.1 Search strategy
A systematic review was carried out using PubMed/Medline and EBSCO databases. The search strategy included medical subject heading (MeSH) terms, as shown in Table 1. Chronologically limited RCTs were considered only. A secondary scan of the lists of retrieved papers was performed to identify any additional RCTs that met the inclusion criteria, as outlined below in subsection 2.2 "study selection". When searching PubMed "title/abstract" was used as a filter, "AB Abstract" was used in the EBSCO database.
Table
1.
Search strings and keywords used to select the studies |
|
 |
 |
Legend:
AUC - area under the curve, DID - daily insulin dose, FCP - fasting C-peptide, HbA1c - glycated hemoglobin, RCT - randomized controlled trial, T1D - type 1 diabetes |
|
2.2 Study selection
This review was set up based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA). PRISMA is an evidence-based set of criteria applicable to the preparation of systematic reviews and meta-analyses; it can be used as a guideline for preparing systematic reviews of research interventions [17]. A structured approach was used to set up the research question for this review, using five components commonly known as the Participants, Interventions, Comparisons, Outcomes, and Study design approach (PICOS) [18]:
- Participants: newly diagnosed T1D patients with standard insulin treatment; no restrictions were placed on gender, age, race, and geographical distribution of the individuals enrolled in the study.
- Interventions: vitamin D oral supplementation; the supplements used were vitamin D, 1-α-hydroxyvitamin D3, ergocalciferol, cholecalciferol, calcitriol, alphacalcidole or 1α,25-dihydroxyvitamin D3.
- Comparisons: placebo and other non-vitamin D interventions.
- Outcome: changes in daily insulin doses (DID, IU/d); changes in glycemic indexes: hemoglobin A1c (HbA1c, %), fasting C-peptide (FCP, nmol/l), and stimulated C-peptide (SCP, (pmol/l), (ng/ml), (nmol/l×120 min)).
- Study design: RCTs.
Studies excluded from the selection were:
- Clinical studies in patients with type 2 diabetes (T2D)
- Animal studies
- Clinical studies in healthy individuals
- Clinical studies in which supplementation was combined with calcium and/or other vitamins
A p-value of <0.05 was considered statistically significant for all included studies.
2.3 Assessment of methodological quality and data extraction
Two authors (G.E. and C.S) extracted independently the detailed information from the selected trials. Qualitative and quantitative information from each study was extracted, including author names, year of study, geographic study location, study size, age of participants, type of exposure (i.e. formulation), duration of study, and dosage of vitamin D supplementation.
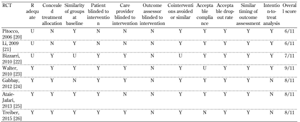
The methodological quality of the included RCTs was assessed by means of 11 predefined criteria based on the updated Cochrane Collaboration Back Review Group (CCBRG) method (Table 2) [19]. The internal validity criteria that refer to the characteristics of the study are related to:
- Selection bias (criteria 1 and 2)
- Performance bias (criteria 4-8)
- Attrition bias (criteria 9 and 11)
- Detection bias (criteria 6 and 10) (Table 2)
Table
2.
Quality assessment of seven randomized controlled trials using the 11 criteria proposed by van Tulder [19] |
|
 |
 |
Legend:
High quality: ≥ 8 out of 11 validity criteria. Low quality: < 8 out of 11 validity criteria [19]. Abbreviations: Y - yes, N - no, U - unknown. |
|
The above criteria were characterized as: "yes", "no" or "unknown". Two authors (G.E. and C.S.) used the assessment instrument independently to evaluate the quality of each study. Whenever there was a disagreement between the two investigators, a third author (M.I.) provided an additional evaluation. Accordingly, each study was assessed as high quality if the total score of positive responses was ≥8 out of 11; it was regarded as low quality if the total score of positive responses was ≤7 out of 11.
3. Results
3.1 Search strategy and study selection
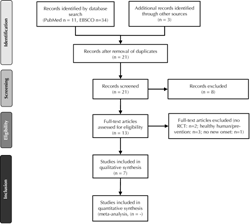
The search strategy and study selection processes were performed according to the "PRISMA" method, as described above. The flow chart for included studies is presented in Figure 1 [14]. Overall, 7 studies were deemed eligible for inclusion in the current analysis [20-26].
 |
 |
Figure 1. PRISMA flow diagram for the selection of studies of this systematic review. The search strategy was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. |
|
The quality assessment of the studies included revealed 4 studies [23-26] of high methodological quality (≥8/11 validity criteria) and 3 studies [20-22] of low quality (≤7/11) [19]. Table 2 shows the result of the quality assessment of the included studies.
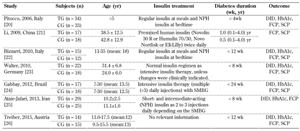
3.2 Description of study characteristics
The study characteristics are summarized in Table 3. The studies were conducted in different geographic locations. Alphacalcidole, cholecalciferol, and calcitriol were the main forms of vitamin D supplementation used in the studies. The time between diagnosis of T1D and initiation of vitamin D intervention varied, ranging between 4 weeks and 1 year. Similarly, the duration of vitamin D supplementation varied between 6 and 24 months.
Table
3.
Basic characteristics of the studies included |
|
 |
 |
Legend:
CG - control group, DID - daily insulin dose, HbA1c - glycated hemoglobin, FCP - fasting C-peptide, SCP - stimulated C-peptide, SMBG - self-monitored blood glucose, TG - treatment group. |
|
3.3 Effect on DID during and after treatment
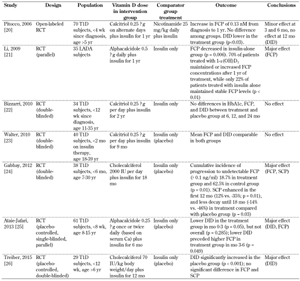
The study by Pitocco et al. (2006) showed that DID was significantly lower in the treatment than in the control group at month 3 and 6 of treatment (p = 0.03), but no effect was seen at 12 months [20]. In the study by Ataie-Jafari et al. (2013), the DID was significantly different between the two groups (p = 0.008) in the between-subject comparison, with lower values for DID in the treatment than in the control group [25]. Furthermore, in the study by Treiber et al. (2015), DID was significantly increased in the control group after 12 months of treatment (p < 0.001), while no change was observed in the treatment group [26]. The results of vitamin D supplementation effects on DID are shown in Table 4.
Table
4.
Assessment of study outcomes (DID, HbA1c, FCP, and SCP) during follow-up, before and after vitamin D supplementation |
|
 |
 |
3.4 Effects on glycemic indices
There was no significant effect on HbA1c levels during or after treatment, but there was an effect on FCP. In the study by Gabbay et al. (2012), the cumulative incidence of progression to undetectable levels of FCP (≤0.1 ng/ml) during the 18 months of monitoring was lower in the treatment than in the control group (p = 0.01) [24]. In the study by Ataie-Jafari et al. (2013), within-subject comparisons showed that the differences in FCP between the treatment group and the control group were highest at 3 and 6 months of treatment (p = 0.049) [25]. FCP levels were reduced in the treatment group compared to those of the control group.
In the study by Li et al. (2009), FCP levels decreased significantly in the control group between baseline and months 6 and 12 of therapy (p = 0.006), but no changes were observed in the treatment group [21]. Also, FCP levels were maintained or increased in vitamin D-treated patients compared to control subjects (p < 0.01). The results of the effect of vitamin D supplementation on the FCP are shown in Table 4.
A positive effect on SCP was found by Gabbay et al. (2012). In this study, SCP increased in the first 12 months (p = 0.01), and showed reduced decline after 18 months in the treatment group compared to the control group (p = 0.03) [24]. The cumulative incidence of progression to undetectable SCP (≤0.1 ng/ml) was observed in 6.2% of the patients in the treatment group and in 37.5% of subjects in the placebo group (p = 0.047). The results of vitamin D supplementation on SCP are shown in Table 4.
4. Discussion
The current review shows that alphacalcidiole, cholecalciferol, and calcitriol are the main forms of vitamin D supplementation examined in patients with newly diagnosed T1D. This is a systematic review including studies of different population characteristics, from different countries (namely Italy, China, Germany, Brazil, Iran, and Austria), and with patients of different ages (children and adults). Moreover, the duration of supplementation varied between 6 to 24 months, and the doses of vitamin D differed between the studies. Although all different types of supplementation were found to be safe, there was a considerable variation in their effectiveness. Another factor that may have led to inconsistencies between the study results is the different quality of the studies included, with some studies below the cutoff for high quality [20-22] and some above [23-26] (Table 2).
4.1 Positive effect on T1D progression after alphacalcidole supplementation
Alphacalcidole supplementation resulted in considerable differences in FCP and DID levels, which was possibly due to the effect of dosage and duration of supplementation [18, 25]. The current findings indicate that alphacalcidole appears to be the ideal form of supplementation in patients with newly diagnosed T1D. It is noteworthy that these are the only existing studies using alphacalcidole supplementation in patients with diabetes. Therefore, more research is required to confirm the effectiveness of this particular treatment through well-designed, high-quality, randomized controlled trials.
4.2 Positive effect on T1D progression after cholecalciferol supplementation
Our review identified considerable differences in the effects of cholecalciferol supplementation on FCP [24], SCP [24], and DID levels [24, 26] in newly diagnosed T1D patients. These differences may be affected by the dosage and duration of cholecalciferol supplementation. For example, the positive effect shown in the study by Gabbay et al. (2012) may be explained by the relatively long period of 18 months (with daily supplementation of 2000 IU) [24], while in the study by Treiber et al. (2015) cholecalciferol was administered at a higher dose (70 IU/kg/day), but for a shorter period of time (12 months) [26].
After 12 months of treatment, cholecalciferol supplementation showed an indirect positive and potentially protective effect on DID, which was due to a negative effect in the control group (increase in DID). Although both studies [24, 26] are of high methodological quality, the positive effects were most pronounced in the first study [24]. These findings agree with previous studies showing that dosages of 2000 IU/day given early in life may have a protective effect, and reduce the risk of T1D [8, 27, 28]. The results indicate that further investigation is required to evaluate whether a daily standardized dose of cholecalciferol equal to 2000 IU for about 6 to 18 months is effective in improving pancreatic β-cell function in newly diagnosed T1D patients.
4.3 Inconsistent effects on T1D progression after calcitriol supplementation
Our review identified that short-term calcitriol supplementation in doses of 0.25 μg/day has no effect on glycemic indices at the end of the treatment [20, 22, 23]. Moreover, only one of the above-mentioned studies was of high methodological quality [23], thus weakening the evidence of the potentially positive effect of calcitriol supplementation in these patients. The dose of supplementation seems to be too small to induce a significant effect. A review by Antico et al. (2012) indicated that the dose of vitamin administered in human studies is insufficient to attenuate the progression of the autoimmune disease [29]. Therefore, more studies are necessary to determine the most effective and safe dose of calcitriol in newly diagnosed T1D patients.
5. Methodological considerations
The key strength of the current review is the sole inclusion of RCTs, a study design which is less likely to be biased and affected by confounding variables. More than half of the studies included were assessed as high quality (4/7), which reinforces reliability of the study outcomes.
The limitations of the current study concern the diversity in ethnicity of the study populations included, in length of treatment, and in type of supplementation used. The studies included supplementations ranging from 6 to 24 months. Long-term follow-up studies of >24 months simply did not exist. Furthermore, factors such as history of diabetes control and baseline vitamin D levels prior to commencement of therapy were not examined in the studies, but could have affected outcomes.
The diversity in study populations regarding comorbidity, medical history, and medications, as well as the differing criteria for patient inclusion and exclusion in the studies are limitations that need to be eliminated in future clinical trials. Future studies should also consider the genetic diversity of the disease as this could be another factor impacting outcome. The human leukocyte antigen (HLA) region has numerous polymorphisms that are among the greatest contributors to the genetic susceptibility to T1D. Although many studies to date include data regarding genes associated with T1D, more research is necessary to reveal the exact mechanisms by which HLA and other associated loci confer T1D susceptibility, and lead to altered disease severity and progression [30].
Beside variations in the study population, the differences in outcomes may also be attributed to factors such as biomarker accuracy and efficiency in diagnosing and predicting T1D [31]. Furthermore, interaction with other medications may also blur the effect of vitamin D in T1D [32, 33], while iron status may further affect the level of HbA1c independent of glycemia [34]. Comorbidities and racial differences may further account for the observed diversity in biomarker levels. Since all currently available glycemic biomarkers have advantages and limitations, a more careful examination of the variables measured may yield better results, better decisions, and better claims for future therapeutic regimens.
Finally, the lack of basic research regarding the biological mechanisms of action required to justify the observed correlation between vitamin D and T1D increases the need for experimental studies. These studies may elucidate the key mechanisms and provide reproducible results that lead to translational medicine and clinical practices in the future.
6. Conclusions
The current analysis showed that vitamin D supplementation has a short-term positive effect on newly diagnosed T1D patients. This has been found in most of the studies irrespective of the heterogeneity of the study characteristics (e.g. differences in type, duration, and dosage of supplementation) and differences in study populations (e.g. regarding age and ethnicity).
In particular, alphacalcidole is a promising form for supplementation therapy as it has positive effects on pancreatic β-cell function. Cholecalciferol is another safe and effective form of supplementation. The effectiveness of calcitriol remains controversial; it is assumed that it is less effective and safe than the other two forms of vitamin D supplements.
Finally, studies have shown evidence that vitamin D intake early in life reduces the risk of T1D, although there is not enough evidence for an association between maternal intake of vitamin D and risk of T1D in the offspring [29, 30]. These results highlight the need for further research in this field. High-quality and long-term RCTs are required to elucidate further the role of vitamin D in pancreatic β-cell function and T1D in both pediatric and adult populations.
Disclosures: The authors reported no conflict of interests.
References
- Harinarayan CV. Vitamin D and diabetes mellitus. Hormones (Athens) 2014. 13(2):163-181. [DOD]
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011. 91(1):79-118. [DOD] [CrossRef]
- Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010. 39(3):481-497. [DOD] [CrossRef]
- Busta A, Alfonso B, Poretsky L. Role of Vitamin D in the Pathogenesis and Therapy of Type 1 Diabetes Mellitus, Type 1 Diabetes - Complications, Pathogenesis, and Alternative Treatments, InTech 2011, Chapter 19, pp 403-422. [DOD]
- Griz LH, Bandeira F, Gabbay MA, Dib SA, Carvalho EF. Vitamin D and diabetes mellitus: an update 2013. Arq Bras Endocrinol Metabol 2014. 58(1):1-8. [DOD] [CrossRef]
- Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am 2014. 43(1):205-232. [DOD] [CrossRef]
- Ritzerfeld M, Klasser M, Mann H. Alfacalcidol in the therapy of renal bone disease. Int J Clin Pharmacol Ther 2001. 39(12):546-550. [DOD] [CrossRef]
- Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr 2005. 135(2):323-325. [DOD]
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999. 69(5):842-856. [DOD]
- National Institute of Health. Vitamin D - Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminDHealthProfessional/. [DOD]
- Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol 2013. 136:321-329. [DOD] [CrossRef]
- Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin D receptor and T cell function. Front Immunol 2013. 4:148. [DOD] [CrossRef]
- Olliver M, Spelmink L, Hiew J, Meyer-Hoffert U, Henriques-Normark B, Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to streptococcus pneumoniae. J Infect Dis 2013. 208:1474-1481. [DOD] [CrossRef]
- George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med 2012. 29(8):e142-e150. [DOD] [CrossRef]
- Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol 2004. 89-90(1-5):121-125. [DOD] [CrossRef]
- Bourlon PM, Billaudel B, Faure-Dussert A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J Endocrinol 1999. 160(1):87-95. [DOD] [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 2009. 6(7):e1000097. [DOD] [CrossRef]
- O'Connor D, Green S, Higgins JP. Defining the review question and developing criteria for including studies. John Wiley and Sons, 2008, pp. 81-94. [DOD]
- van Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976) 2003. 28(12):1290-1299. [DOD]
- Pitocco D, Crino A, Di Stasio E, Manfrini S, Guglielmi C, Spera S, Anguissola GB, Visalli N, Suraci C, Matteoli MC, et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI). Diabet Med 2006. 23(8):920-923. [DOD] [CrossRef]
- Li X, Liao L, Yan X, Huang G, Lin J, Lei M, Wang X, Zhou Z. Protective effects of 1-alpha-hydroxyvitamin D3 on residual beta-cell function in patients with adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev 2009. 25(5):411-416. [DOD] [CrossRef]
- Bizzarri C, Pitocco D, Napoli N, Di Stasio E, Maggi D, Manfrini S, Suraci C, Cavallo MG, Cappa M, Ghirlanda G, et al. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care 2010. 33(9):1962-1963. [DOD] [CrossRef]
- Walter M, Kaupper T, Adler K, Foersch J, Bonifacio E, Ziegler AG. No effect of the 1alpha,25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care 2010. 33(7):1443-1448. [DOD] [CrossRef]
- Gabbay MA, Sato MN, Finazzo C, Duarte AJ, Dib SA. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual beta-cell function in new-onset type 1 diabetes mellitus. Arch Pediatr Adolesc Med 2012. 166(7):601-607. [DOD] [CrossRef]
- Ataie-Jafari A, Loke SC, Rahmat AB, Larijani B, Abbasi F, Leow MK, Yassin Z. A randomized placebo-controlled trial of alphacalcidol on the preservation of beta cell function in children with recent onset type 1 diabetes. Clin Nutr 2013. 32(6):911-917. [DOD] [CrossRef]
- Treiber G, Prietl B, Frohlich-Reiterer E, Lechner E, Ribitsch A, Fritsch M, Rami-Merhar B, Steigleder-Schweiger C, Graninger W, Borkenstein M, Pieber TR. Cholecalciferol supplementation improves suppressive capacity of regulatory T-cells in young patients with new-onset type 1 diabetes mellitus - A randomized clinical trial. Clin Immunol 2015. 161(2):217-224. [DOD] [CrossRef]
- Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001. 358:1500-1503. [DOD] [CrossRef]
- Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child 2008. 93(6):512-517. [DOD] [CrossRef]
- Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev 2012. 12(2):127-136. [DOD] [CrossRef]
- Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med 2012. 2(1):a007732. [DOD] [CrossRef]
- Wright LA, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr 2012. 25:141-148. [DOD] [CrossRef]
- Albright ES, Ovalle F, Bell DS. Artificially low hemoglobin A1c caused by use of dapsone. Endocr Pract 2002. 8(5):370-372. [DOD] [CrossRef]
- Uzu T, Hatta T, Deji N, Izumiya T, Ueda H, Miyazawa I, Kanasaki M, Isshiki K, Nishio T, Arimura T. Target for glycemic control in type 2 diabetic patients on hemodialysis: effects of anemia and erythropoietin injection on hemoglobin A(1c). Ther Apher Dial 2009. 13(2):89-94. [DOD] [CrossRef]
- El-Agouza I, Abu Shahla A, Sirdah M. The effect of iron deficiency anaemia on the levels of haemoglobin subtypes: possible consequences for clinical diagnosis. Clin Lab Haematol 2002. 24(5):285-289. [DOD] [CrossRef]
|