Case Report
| Rev Diabet Stud,
2005,
2(2):92-96 |
DOI 10.1900/RDS.2005.2.92 |
Cholesterol Emboli Syndrome in Type 2 Diabetes: The Disease History of a Case Evaluated with Renal Scintigraphy
Giorgina B. Piccoli1, Antonella Sargiotto2, Manuel Burdese1, Loredana Colla1, Donatella Bilucaglia1, Andrea Magnano1, Valentina Consiglio1, Giuseppe Piccoli1, Giuseppe Picciotto2
1Chair of Nephrology, Department of Internal Medicine, University of Turin, Corso Bramante 86-88, 10126 Torino, Italy.
2S.C. Nuclear Medicine, A.S.O. "S. Giovanni Battista" - Turin, Italy.
Address correspondence to: Giorgina B. Piccoli, e-mail: giorgina.piccoli@unito.it.
Keywords: cholesterol crystal embolism, type 2 diabetes, renal artery stenosis, chronic kidney disease
Abstract
BACKGROUND: Cholesterol crystal emboli syndrome (CCE) is an emerging disease, whose progression reflects the currently observed increase in cardiovascular diseases. Diagnostic criteria shifted from pathological to clinical criteria: creatinine increase, skin lesions, recent endovascular interventions and severe vasculopathy). Diabetes, hypertension and diffuse vascular disease are inter-linked, major risk factors. The role of imaging techniques in the diagnosis and treatment of the disease has been little investigated thus far. The AIM of this report is to describe a case exemplifying the potentials for renal scintigraphy in CCE, an emerging disease in type 2 diabetic patients. THE CASE: a 75 year-old, type 2 diabetic for over 15 years, obese, hypertensive white man was referred to the Nephrology Unit after an acute coronary syndrome. Stenosis of the left renal artery was diagnosed from the angiography. Serum creatinine (baseline: 1.9 mg/dl) increased after multiple angioplasties to 3.3 mg/dl, then slowly returned towards baseline (2.2 mg/dl), but rose, on referral, to 3.9 mg/dl, with an increase in acute phase reactants and peripheral livedo reticularis, a picture highly suggestive of CCE. The first renal scintiscan showed a reduction of the parenchymal phase, and a non-homogeneous parenchymal pattern in the right dominant kidney. The patient was started on corticosteroid therapy with a prompt decrease in creatinine; four days later (creatinine 2.5 mg/dl) a second scintiscan showed an improvement of the peak time and of the radionuclide parenchymal transit, and was further confirmed two months later (creatinine 2.2 mg/dl). No modification was detected in the left kidney, presumably mechanically "protected" from the cholesterol shedding by the stenosis. CONCLUSIONS: This is the first description of an imaging demonstration of the morpho-functional substratum to the rapid clinical response of corticosteroid therapy in a case of CCE and type 2 diabetes, underlining the potential of 99mTc-MAG3 dynamic scintiscan in this disease.
Introduction
Cholesterol crystals emboli syndrome (CCE) is an emerging disease characterised by the fragmentation and the distal shedding of atherosclerotic plaque, with an initial immediate ischemic effect (mechanical phase) and a subsequent inflammatory reaction (inflammatory phase), and with a further increase in ischemic lesions [1-3]. The fragmentation of the plaque may be spontaneous or iatrogenic, the latter being more common and usually related to recent endovascular procedures [1-6]. The major clinical signs and symptoms are related to ischemic lesions in the tissues with higher vascularization, such as skin, kidney and gastro-enteric traits; however, there is no single tissue spared by CCE.
CCE may be considered as a disease of progress: its incidence increased with the progressive ageing of the overall population, and with the concomitant increase of the burden of chronic diseases of the elderly, including diabetes, chronic kidney disease and diffuse vasculopathy. The concomitant advances in interventional angio-radiographic techniques allowed the extension of the indications of percutaneous revascularization also in elderly patients and in patients with concomitant co-morbidities, such as diabetes or chronic kidney disease; in this context, severe side effects, such as CCE may be expected to increase [1-3].
While the first observations of CCE were identified in autopsy studies, and also in vivo the demonstration of cholesterol crystals in the microvasculature was initially required for diagnosis, in recent years, taking also into account the frequent false-negatives in the case of skin lesions and the invasiveness of parenchymal organ biopsies, clinical criteria are being increasingly employed. Clinical criteria are mainly derived, with a few changes, from the classical ones originally described by Mayo and co-workers (recent endovascular intervention, acute or sub-acute kidney failure, skin lesions), usually accompanied by signs of acute inflammation [1-6].
Diabetes, hypertension and diffuse vascular disease are major risk factors for both iatrogenic and spontaneous CCE; among them, diabetes mellitus, often in the context of the "metabolic syndrome", is probably the most important risk element [4-6].
From the nephrological point of view, the clinical feature may be an acute renal function impairment, usually following an endovascular intervention, or a smouldering and sub-acute decrease of kidney function, due to continuous, "spontaneous" shedding of cholesterol emboli. In patients with pre-existing chronic kidney disease or diabetes, the diagnosis may be particularly difficult, due to the "background noise" of diabetic nephropathy, a progressive disease, often with a capricious progression pattern.
The role of imaging tests is usually considered as having great potential for ensuring the accuracy of diagnosis, by demonstrating the presence of diffuse vascular disease and of atherosclerotic plaques (in particular if ulcerated). However, at the kidney level, ultrasounds are non-specific, usually demonstrating either small vascular-induced shrunken kidneys or intense sinusal sclerolipomatosis resulting from microvascular disease. In contrast, due to the lack of precise correspondence between structure and function, this imaging technique is not appropriate for pursuing therapeutic effects or clinical developments.
In this context, renal dynamic scintiscan with tubular tracer may represent a useful imaging technique in the clinical setting. The reasons are that it enables a suggestive, even though non-specific, picture of microlacunar involvement of the kidney parenchyma (patchy uptake), and facilitates the detection of functional changes conducive to microvascular changes. The latter are bilateral cortical retention with delayed excretion resulting from prolonged parenchymal transit time.
This report presents the case of a type 2 diabetic patient who exhibits a series of major risk factors, which are considered to beresponsible for the formation of late cardiovascular effects (diabetes, obesity, hypertension, diffuse vascular disorders), who developed a CCE after angioplasty and coronary stenting, and who responded well to aggressive support therapy and to steroids.
The novelty in the documentation of this case is mainly due to the use of the renal dynamic scintigraphic patterns, recorded in the different phases of the disease, before and after successful steroid therapy to support diagnosis and to document therapeutic response.
The case
A 75 year-old active, Caucasian man, father of a young colleague, came to the Nephrology Outpatient Unit for a first medical control. He had been affected by type 2 diabetes for at least 15 years, was obese (BMI: 32), but otherwise in good clinical condition (Karnofski score: 100; SGA: 1), working full-time on a self-employed basis as financial consultant, and had never been hospitalized until two months previously.
In April 2004, he was hospitalized in an emergency for an acute coronary syndrome. On hospitalization, chronic kidney disease was diagnosed for the first time. On that occasion, his renal functional data were: serum creatinine 1.9 mg/dl, creatinine clearance 49.3 ml/min; no proteinuria or hematuria were present in the urinary dipstick test. Glycemic control was good (HbA1c: 7.5%), moderate dyslipidemia was also present (total Cholesterol 240 mg/dl, HDL 24 mg/dl; triglycerides: 228 mg/dl).
After coronarography and multiple angioplasties (stenting in left anterior descending and right coronary arteries), serum creatinine rose to 3.3 mg/dl and then slowly returned towards baseline (nadir 2.2 mg/dl). During the angiographic evaluation, left renal artery stenosis (>75%) was also diagnosed. However, the patient refused to undergo a third arteriography and was discharged with advice to start nephrological follow-up examinations.
On referral to the Nephrology Unit, (14 days after the last contrastographic procedure), serum creatinine had suddenly increased to 3.9 mg/dl. Moderate leukocytosis and eosinophilia was observed in several blood tests (WBC: 12,390/mmc, eosinophils: 930/mmc), with a high erythrocyte sedimentation rate (ESR: 41 mm/h) and C-reactive protein (9 mg/l); serum complement was in the normal range. On clinical control, peripheral livedo reticularis was detectable in both feet; no other visceral or neurological signs of disease were present. The timing pattern of renal function impairment was very likely to have been caused by cholesterol embolisation (subacute renal functional impairment within one month after coronarography). All major clinical criteria as well as ancillary elements, indicative of CCE, were indeed present: these are sub-acute kidney failure, recent angiography, skin lesions, together with an increase in acute phase reactants in a patient with severe and diffuse atherosclerotic vascular disease [4].
However, taking into account the presence of severe left renal artery stenosis, detected in the recent angiography, we ruled out the possibility that the increase in serum creatinine was due to a complete obstruction of the left renal artery by using renal dynamic scintigraphy (using a tubular tracer, 99mTc- MAG3).
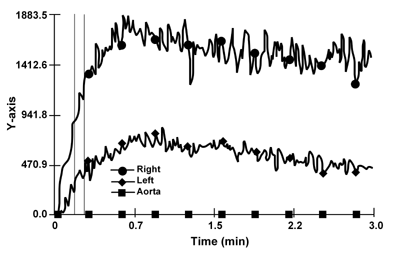
The result of the imaging test is depicted in Figure 1. The right side shows a reduction of the uptake phase with a non-homogeneous, patchy parenchymal pattern and cortical retention in a well-perfused kidney, compatible with an ischemic insult, as indeed a hallmark of cholesterol emboli syndrome, as well as of acute toxic damage, as it may occur after contrast media. However, apart from the clinical picture, the timing of the serum creatinine increase was typical for CCE, since acute contrast media toxicity usually occurs immediately after the procedure. Interestingly, the pattern of the left kidney, supplied by the stenotic artery, was non-specific (Figure 1).
 |
 |
Figure 1. 99 mTc-MAG3 renal scintirenography. The parenchymal curve of the right kidney shows delayed peak time and prolonged excretion. The parenchymal phase of the left kidney is poor, according to the diagnosis of left artery stenosis. |
|
The patient was started on corticosteroid therapy (methylprednisolone 300 mg intravenously for 3 consecutive days, followed by oral therapy with prednisone 25 mg, tapered within two months). After starting this therapy, the serum creatinine levels promptly decreased together with acute phase reactants. Peripheral livedo reticularis disappeared within two days from the start of corticosteroid therapy. Glycemic control was carefully monitored; apart from the need for careful self-management of the insulin therapy, no severe side effects occurred.
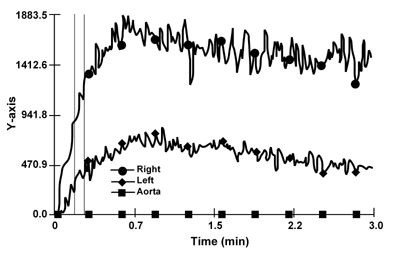
A second renal scintiscan (Figure 2) was performed four days after the start of corticosteroid therapy (when serum creatinine had already decreased to 2.5 mg/dl), to investigate the possible changes of morpho-functional patterns, concomitant to the prompt clinical and laboratory response. An improvement of the peak time and of radionuclide excretion in the right kidney was observed in this second examination. The pattern of the kidney with the stenotic artery, presumably mechanically protected by the shedding of cholesterol emboli, was unchanged.
 |
 |
Figure 2. The second 99 mTc MAG3 renal scintigraphy shows a normalisation of the peak time and a significant improvement in the transit and excretion of the radionuclide in the right kidney. |
|
The positive clinical and imaging response was confirmed two months later, with a third scintigraphy control, showing the same pattern as the second one; serum creatinine was stable at 2.2 mg/dl.
Conclusions
This case is almost paradigmatic for CCE, an increasingly recognized cause for acute and sub-acute renal failure in patients affected by severe vascular disease; our patient had all the major signs of CCE syndrome, both regarding risk factors (diabetes, diffuse vascular disease, hyper-tension, ischemic cardiopathy) and clinical presentation (kidney function impairment, skin lesions, anamnesis of endovascular intervention, together with acute inflammatory signs) [1-9].
Taking into account the patient's good baseline clinical conditions, and the severity of kidney function impairment, he was treated with intravenous and oral steroids, a promising therapy for both the control of systemic symptoms and stabilization of kidney function, which can be regarded as being linked to its anti-inflammatory activity, active against the second ("mechanical") phase of the disease [10-15]. The treatment of our patient was successful, allowing a return of serum creatinine levels towards baseline, and a rapid and complete reversal of acute phase reaction signs and of skin lesions. However, the use of steroids, although not yet established, is not a novel approach, and the clinical response, even if favorable in a very short time, corresponds to several other case reports or case series [10-15].
The novelty in the case refers to the fact that this is, to our knowledge, the first imaging demonstration of the morpho-functional substratum to the rapid clinical response to corticosteroid therapy in CCE with diabetes, which has already been described in several other settings with different treatment schedules [10-15].
While it is impossible to propose general laws on single cases, this report suggests that 99mTc-MAG3 dynamic scintiscan may be an important tool in the diagnosis and follow-up control of renal involvement in CCE. This valuable and flexible diagnostic tool may be of particular interest in cases, in which the "background noise" of pre-existing diabetes and nephropathy may hinder a timely diagnosis and the demonstration of the morpho-functional substratum of the beneficial effect of therapy may help in balancing risks and benefits (as in the case of corticosteroids in diabetics).
We regard it to be worth carrying out further investigations on a larger scale to evaluate the full potential of this imaging technique in this potentially severe, emerging disease.
References
- Darsee JR. Cholesterol embolism: the great masquerader. Southern Med J 1979. 72:174-180. [DOD]
- Moldveen-Geronimus J, Merriem JC. Cholesterol embolisation: from pathologic curiosity to clinical entity. Circulation 1967. 35:1360-1366. [DOD]
- Kassirer JP. Atheroembolic renal disease. New Engl J Med 1969. 280:812-818. [DOD]
- Mayo RR Swartz RD. Redefining the incidence of clinically detectable atheroembolism. Am J Med 1996. 100:524-529. [DOD] [CrossRef]
- Belenfant X, Meryer A, Jacquot C. Supportive treatment improves survival in multivisceral cholesterol crystal embolism. Am J K Dis 1999. 33:840-850. [DOD]
- Fukumoto Y, Tsutsui H, Tsuchihashi M, Masumoto A, Takeshita A. For the Cholesterol Embolism Study (CHEST) investigators. J Am Coll Cardiol 2003. 42:211-216. [DOD] [CrossRef]
- Fine MJ, Kapoor W, Falanga V. Cholesterol crystal embolization: A review of 221 cases in the English literature. Anguology 1987. 38:769-784. [DOD]
- Scolari F, Ravani P, Pola A, Guerini S, Cubani R, Movilli E, Savoldi S, Malcerti F, Maiorca R. Predictors of renal and patient outcomes in atheroembolic renal disease: a prospective study. J Am Soc Nephrol 2003. 14:1584-1590. [DOD] [CrossRef]
- Thadhani RI, Camargo CA, Xavier RJ, Fang LST, Bazari H. Atheroembolic renal failure after invasive procedures: natural history based on 52 histologically proven cases. Medicine 1995. 74: 350-358. [DOD] [CrossRef]
- Scoble JE. Is nihilism in the treatment of atheroembolic disease at an end? Am J K Dis 1999. 33:975-976. [DOD]
- Hasegawa M, Kawashima S, Shikano M, Hasegawa H, Tomita M, Murakami K, Kushimoto H, Katsumata H, Toba T, Oohashi A, Hiramitsu S, Matsunaga K. The evaluation of corticosteroid therapy in conjunction with plasma exchange in the treatment of cholesterol embolic disease. Am J Nephrol 2000. 20:263-267. [DOD] [CrossRef]
- Cappiello RA, Espinoza LR, Adelman H, Aguillar J, Vasy FB, Germain BF. Cholesterol embolism: a pseudovasculitic syndrome. Semin Arthritis and Rheumatism 1989. 18: 24-26. [DOD]
- Boero R, PIgnataro A, Rollino C, Quarello F. Do corticosteroids improbe survival in acute renal failure due to cholesterol atheroembolism? Nephrol Dial Transplant 2000. 15:441. [DOD]
- Espejo SB, Herrero JC, Torres A, Martinez A, Gutierrez E, Morales E, Gonzalers E, Bueno B, Valentin MO, Praga M. Immunoallergic interstitial nephritis vs cholesterol atheroembolism. Differentiating characteristics. Nefrologia 2003. 23:125-130. [DOD]
- Takahashi T, Konta T, Nishida W, Igarashi A, Ichikawa K, Kubota I. Renal cholesterol embolic disease effectively treated with steroid pulse therapy. Intern Med 2003. 42:1206-1209. [DOD]
|