Short Review
| Rev Diabet Stud,
2006,
3(2):72-75 |
DOI 10.1900/RDS.2006.3.72 |
Th17 Cells in Inflammatory Conditions
Anne Cooke
Department of Pathology, University of Cambridge, Tennis Court Rd., Cambridge CB21QP, United Kingdom, e-mail: ac@mole.bio.cam.ac.uk.
Keywords: inflammatory disease, Th cell, regulatory T cell, Th17 cell, IL-17, IL-23, type 1 diabetes
Abstract
CD4+ T cells have been subdivided into different subsets, largely on the basis of the cytokines they produce. These subsets include Th1, Th2 and regulatory T cells. Recently, another population of T cells have been described, namely Th17, which are characterized by their production of IL-17. Two other important cytokines, which are related to each other, are associated with the development of Th cells, namely IL-12 and IL-23. While IL-12 plays a key role in the differentiation of naïve T cells to Th1 cells, IL-23 promotes the expansion of Th17 cells. IL-12 and IL-23 are heterodimers with a shared subunit, p40. They furthermore bind to receptors which have unique and shared subunits. Several previous studies have evaluated the role of IL-12 in inflammatory diseases on the basis of p40. Therefore a reevaluation of the role of IL-12 and Th1 cells in a range of inflammatory conditions has been carried out. This new wave of studies has resulted in the recognition of the role of IL-23 and Th17 cells in inflammatory conditions, such as arthritis and inflammatory bowel disease. There is also the speculation about a possible role in type 1 diabetes.
Introduction
CD4+ T cells have been subdivided into a range of different subsets on the basis of the cytokines they produce and the functions they perform. Th1, Th2 and regulatory T cells (Tregs) have been well characterized with respect to factors influencing their development, the cytokines they produce and their respective roles in response to pathogens, tumors and self-antigens [1-7]. Another population of Th cells, Th17, characterized by their production of the cytokine IL-17, has been described [8], and it is only recently that factors determining their generation have been identified [9, 10]. The role of Th17 cells in mediating autoimmune pathology is also now becoming recognized, interestingly in events that were previously thought to be Th1-mediated. As type 1 diabetes is thought to be a Th1-mediated disease it would be appropriate to examine the involvement of Th17 in beta cell destruction. The role of these cells, however, in type 1 diabetes remains to be clarified.
The IL-17 cytokine family
The IL-17 produced by Th17 cells is IL-17A and is one of the six family members of related cytokines [11]. Of the other family members, IL-17B, C, D, E and F only IL-17E and F are known to also be made by CD4+ T cells. IL-17E, which is also known as IL-25, is associated with Th2 responses and the immune response against helminths [12, 13]. IL-17A is regarded as a pro-inflammatory cytokine capable of eliciting the production of other inflammatory cytokines and chemokines such as IL-6, IL-8, GM-CSF and MCP-1 by fibroblasts, endothelial and epithelial cells [14-19]. Elevated levels of IL-17 have been detected in rheumatoid synovium and have been associated with multiple sclerosis, psoriasis and systemic lupus erythematosus.
Factors influencing Th17 development and differentiation
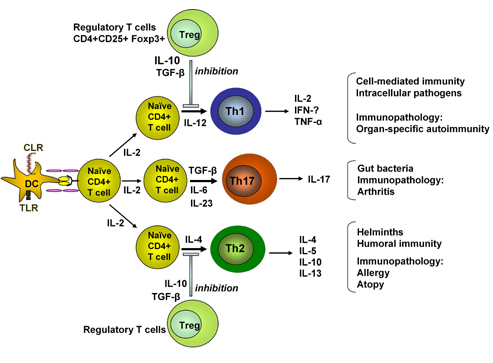
It is well known that IL-12 regulates Th1 while IL-4 regulates Th2 differentiation [7]. Recent studies have clarified the cytokine-related requirements for Th17 differentiation. These elegant studies show that TGF-β and IL-6 are required for naïve CD4+ T cells to differentiate into Th17 cells [9, 10]. This differentiation is facilitated by the absence of IFN-γ and IL-4. As TGF-β has usually been associated with the development of Treg cells [20] and the inhibition of Th1 and Th2 cell differentiation [21], it is interesting to note that in the presence of an inflammatory cytokine, such as IL-6, the inhibition of Treg cell development and differentiation of naïve CD4+ Tcells into Th17 could be observed [9]. This data confirms the view that Th17 cells are a completely separate lineage of CD4+ T cells (Figure 1).
 |
 |
Figure 1. Functional development and activity of Th cell subpopulations. Activation of dendritic cells (DC) follows interaction of pattern recognition receptors such as C type lectin receptors (CLR) or toll like receptors (TLR) with molecules on the surface of microorganisms. Antigen presentation to naïve T cells results in the development of Th1, Th2 or Th17 cells depending on the cytokine milieu. Cells of the innate immune system, e.g. DC and NKT cells, are the source of promoting cytokines, IL-4, IL-6, TGF-β and IL-12. IL-23 promotes the expansion rather than the development of Th17 cells. The cytokines which are made would depend on the antigenic stimulus. The activity of Th cell subpopulations can be regulated by Tregs which may include naturally arising Foxp3-expressing Tregs. |
|
It is known that the signal transducer and activator of transcription (STAT)-4 and the transcription factor T-bet are essential for development of Th1 while STAT-6 and GATA-3 are needed for Th2 development. However, only recently it could be shown that STAT-3 is also involved in Th17 development together with the suppression of cytokine signaling (Socs) 3, which acts as a regulator of IL-23-induced STAT-3 phosphorylation and Th17 development.
IL-23 has also been shown to regulate IL-17 production and to promote the expansion of Th17 cells [22, 23]. It is a heterodimeric cytokine comprised of p40 and p19 subunits. The p40 subunit is also found in IL-12. The receptors for IL-23 and IL-12 share a subunit, IL-12 Rβ1. The IL-12Rβ1 subunit combines with IL-23R to give the functional IL-23 receptor and, together with IL-12Rβ2, to give a functional IL-12R. It can be seen that this subunit sharing by these cytokines and their receptors could have been a source of confusion in assigning a role for IL-12 and Th1 cells in certain pathological conditions. Early studies suggested that effects on inflammatory responses can be observed following the blockade of p40 with antibody or its targeted mutation. These results were interpreted as demonstrating an involvement of IL-12 and correspondingly Th1 cells in the pathogenesis of inflammatory conditions. However, subsequent studies showed that the specific targeting of IL-23 resulted in the alleviation of several inflammatory conditions [24-26], while the lack of IL-12 in some situations actually exacerbated inflammation [24]. In summary, these studies emphasized the potential importance of Th17 cells in inflammatory responses.
Th17 cells and autoimmune disease
IL-17 levels are elevated in a range of inflammatory conditions including systemic sclerosis, psoriasis and rheumatoid arthritis synovium [27-31]. Collagen induced arthritis (CIA) and experimental induced encephalomyelitis (EAE) were thought to be Th1-mediated autoimmune diseases. As discussed above, this assumption arose in part from experiments that did not distinguish between effects on IL-12 or IL-23. More recently it has been shown that these two experimentally induced autoimmune conditions as well as some others are mediated by Th17 cells. The absence of IL-6, which is needed for Th17 development, protected mice against both EAE and CIA [32-35]. Neutralization of IL-17 by specific antibody treatments in vivo has been shown to prevent the induction of EAE and the deficiency in IL-23 has been shown to protect mice against both EAE and CIA. The involvement of Th17 cells in mediating pathology in EAE has recently been beautifully demonstrated by the work of Kuchroo and colleagues [9].
There is little information, as yet, regarding the role of IL-17 and Th17 cells in type 1 diabetes. The ability of IL-17 to induce inducible nitric oxide synthase (iNOS) in chondrocytes [36] could be observed to occur in mouse islets exposed to this cytokine [37]. A potential role for Th17 cells in the exacerbation of diabetes is suggested by the observation that IL-23 induces diabetes in mice if co-administered with sub diabetogenic multiple low doses of streptozotocin [38]. Whether Th17 cells and cytokines IL-23 and IL-17 play a role in the spontaneous onset of diabetes remains to be clarified.
Conclusion
There has been recent interest in the role of a distinct lineage of Th cells, Th17 cells, in autoimmune pathology. Several autoimmune conditions, previously assumed to be Th1-mediated, have now been shown to involve Th17 cells. The different subpopulations of Th cells have distinct functions and have evolved to combat infections of historical importance. Figure 1 shows that Th17 cells may have a specific role in combating certain bacterial gut infections, thus complementing the activities of Th1 and Th2 cells in their responses against intracellular pathogens and helminths, respectively. The other side of the coin is that the same cell populations are associated with inflammatory responses that can be damaging to the host. In the presence of tight regulatory networks, host pathology should be controlled. There is increasing interest in the ways in which infections may influence the establishment of such regulatory networks.
Acknowledgments:
I would like to thank Dr. Paola Zaccone for her input in the generation of this manuscript.
References
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989. 7:145-173. [DOD] [CrossRef]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today 1988. 9(9):268-274. [DOD] [CrossRef]
- Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol 2004. 16(2):203-208. [DOD] [CrossRef]
- Fitch FW, McKisic MD, Lancki DW, Gajewski TF. Differential regulation of murine T lymphocyte subsets. Annu Rev Immunol 1993. 11:29-48. [DOD] [CrossRef]
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol 2005. 6(4):331-337. [DOD] [CrossRef]
- Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity 1995. 2(6):665-675. [DOD] [CrossRef]
- O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 1998. 8(3):275-283. [DOD] [CrossRef]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005. 6(11):1123-1132. [DOD] [CrossRef]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006. 441(7090):235-238. [DOD] [CrossRef]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006. 24(2):179-189. [DOD] [CrossRef]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004. 21(4):467-476. [DOD] [CrossRef]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 2006. 203(4):1105-1116. [DOD] [CrossRef]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 2006. 203(4):843-849. [DOD] [CrossRef]
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol 2002. 71(1):1-8. [DOD]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 1996. 183(6):2593-2603. [DOD] [CrossRef]
- Cai XY, Gommoll CP Jr, Justice L, Narula SK, Fine JS. Regulation of granulocyte colony-stimulating factor gene expression by interleukin-17. Immunol Lett 1998. 62(1):51-58. [DOD] [CrossRef]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 1998. 160(7):3513-3521. [DOD]
- Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol 1999. 162(4):2347-2352. [DOD]
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol 1995. 155(12):5483-5486. [DOD]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003. 198(12):1875-1886. [DOD] [CrossRef]
- Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol 2002. 2(1):46-53. [DOD] [CrossRef]
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003. 278(3):1910-1914. [DOD] [CrossRef]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005. 201(2):233-240. [DOD] [CrossRef]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 2003. 198(12):1951-1957. [DOD] [CrossRef]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003. 421(6924):744-748. [DOD] [CrossRef]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006. 116(5):1310-1316. [DOD] [CrossRef]
- Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol 1998. 111(4):645-649. [DOD] [CrossRef]
- Albanesi C, Scarponi C, Cavani A, Federici M, Nasorri F, Girolomoni G. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol 2000. 115(1):81-87. [DOD] [CrossRef]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 1999. 103(9):1345-1352. [DOD]
- Kurasawa K, Hirose K, Sano H, Endo H, Shinkai H, Nawata Y, Takabayashi K, Iwamoto I. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum 2000. 43(11):2455-2463. [DOD] [CrossRef]
- Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 1999. 42(5):963-970. [DOD] [CrossRef]
- Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol 1998. 28(7):2178-2187. [DOD] [CrossRef]
- Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, De Benedetti F, Poli V, Ciliberto G. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med 1998. 187(4):461-468. [DOD] [CrossRef]
- Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M, Kishimoto T. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci U S A 1998. 95(14):8222-8226. [DOD] [CrossRef]
- Okuda Y, Sakoda S, Bernard CC, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol 1998. 10(5):703-708. [DOD] [CrossRef]
- Martel-Pelletier J, Mineau F, Jovanovic D, Di Battista JA, Pelletier JP. Mitogen-activated protein kinase and nuclear factor kappaB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: possible role of transactivating factor mitogen-activated protein kinase-activated proten kinase (MAPKAPK). Arthritis Rheum 1999. 42(11):2399-2409. [DOD] [CrossRef]
- Miljkovic D, Cvetkovic I, Momcilovic M, Maksimovic-Ivanic D, Stosic-Grujicic S, Trajkovic V. Interleukin-17 stimulates inducible nitric oxide synthase-dependent toxicity in mouse beta cells. Cell Mol Life Sci 2005. 62(22):2658-2668. [DOD] [CrossRef]
- Mensah-Brown EP, Shahin A, Al-Shamisi M, Wei X, Lukic ML. IL-23 leads to diabetes induction after subdiabetogenic treatment with multiple low doses of streptozotocin. Eur J Immunol 2006. 36(1):216-223. [DOD] [CrossRef]
This article has been cited by other articles:

|
Gut microbiota and type 1 diabetes
Vaarala O
Rev Diabet Stud 2012. 9(4):251-259
|
|

|
Pathogenic Mechanisms in Type 1 Diabetes: The Islet is Both Target and Driver of Disease
Graham KL, Sutherland RM, Mannering SI, Zhao Y, Chee J, Krishnamurthy B, Thomas HE, Lew AM, Kay TW
Rev Diabet Stud 2012. 9(4):148-168
|
|

|
A phase 2, 24-week, randomized, placebo-controlled, double-blind study examining the efficacy and safety of an anti-interleukin-12 and -23 monoclonal antibody in patients with relapsing-remitting or secondary progressive multiple sclerosis
Vollmer TL, Wynn DR, Alam MS, Valdes J
Mult Scler 2011. 17(2):181-191
|
|

|
Imbalance in T-cell and cytokine profiles in patients with relapsing-remitting multiple sclerosis
Mikulkova Z, Praksova P, Stourac P, Bednarik J, Michalek J
J Neurol Sci 2011. 300(1-2):135-141
|
|

|
Immunopathology of the human pancreas in type-I diabetes
Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK
Semin Immunopathol 2011. 33(1):9-21
|
|
|
Heat-killed Trypanosoma cruzi induces acute cardiac damage and polyantigenic autoimmunity
Bonney KM, Taylor JM, Daniels MD, Epting CL, Engman DM
PLoS One 2011. 6(1):e14571
|
|

|
Th17 cells: positive or negative role in tumor?
Ji Y, Zhang W
Cancer Immunol Immunother 2010. 59(7):979-987
|
|

|
Type 1 diabetes in BioBreeding rats is critically linked to an imbalance between Th17 and regulatory T cells and an altered TCR repertoire
van den Brandt J, Fischer HJ, Walter L, Hünig T, Klöting I, Reichardt HM
J Immunol 2010. 185(4):2285-2294
|
|

|
Unregulated IL-23/IL-17 immune response in autoimmune diseases
Costa VS, Mattana TC, da Silva ME
Diabetes Res Clin Pract 2010. 88(3):222-226
|
|

|
Numerical defects in CD8(+)CD28(-) T-suppressor lymphocyte population in patients with type 1 diabetes mellitus and multiple sclerosis
Mikulkova Z, Praksova P, Stourac P, Bednarik J, Strajtova L, Pacasova R, Belobradkova J, Dite P, Michalek J
Cell Immunol 2010. 262(2):75-79
|
|

|
Functional alterations of proinflammatory monocytes by T regulatory cells: implications for the prevention and reversal of type 1 diabetes
Sia C, Hänninen A
Rev Diabet Stud 2010. 7(1):6-14
|
|

|
Nephropathic complication of type-2 diabetes is following pattern of autoimmune diseases?
Arababadi MK, Nosratabadi R, Hassanshahi G, Yaghini N, Pooladvand V, Shamsizadeh A, Hakimi H, Derakhshan R
Diabetes Res Clin Pract 2010. 87(1):33-37
|
|

|
Flt3-ligand decreases Th17 cells and SOCS proteins in the lung of house dust mite-sensitized and challenged mice
McGee HS, Stallworth AL, Agrawal T, Shao Z, Lorence L, Agrawal DK
Am J Respir Cell Mol Biol 2009. Nov 20, epub ahead of print
|
|

|
Chronic inflammation as a manifestation of defects in immunoregulatory networks: implications for novel therapies based on microbial products
Bottasso O, Docena G, Stanford JL, Grange JM
Inflammopharmacology 2009. 17(4):193-203
|
|

|
Serum antibodies and cytokines in C4-deficient mice and their responses to exercise
Visetnoi S, Chawengkirttikul R, Chaiyaroj SC, Kitiyanant Y, Pholpramool C
Asian Pac J Allergy Immunol 2009. 27(4):199-206
|
|

|
Microarray analysis reveals difference in gene expression profiles of hair and wool sheep infected with Haemonchus contortus
Mackinnon KM, Burton JL, Zajac AM, Notter DR
Vet Immunol Immunopathol 2009. 130(3-4):210-220
|
|

|
Cytokines in the beginning stages of the immune response
Adler G
Reumatologia 2009. 47(4):230-235
|
|

|
An orally bioavailable synthetic analogue of an active DHEA metabolite reduces established disease in rodent models of rheumatoid arthritis
Offner H, Firestein GS, Boyle DL, Pieters R, Frincke JM, Garsd A, White SK, Reading CL, Auci DL
J Pharmacol Exp Ther 2009. 329(3):1100-1109
|
|

|
TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin
Castellanos-Rubio A, Santin I, Irastorza I, Castaño L, Carlos Vitoria J, Ramon Bilbao J
Autoimmunity 2009. 42(1):69-73
|
|

|
Impact of protective IL-2 allelic variants on CD4+ Foxp3+ regulatory T cell function in situ and resistance to autoimmune diabetes in NOD mice
Sgouroudis E, Albanese A, Piccirillo CA
J Immunol 2008. 181(9):6283-6292
|
|

|
Immunopathology of type 1 diabetes mellitus
Gallardo CD, Guzman MA
Arch Alergia Immunol Clin 2008. 39(4):151-160
|
|

|
Down-Regulation of Allergic Responses in Conditions of Experimental Diabetes: A Role for Glucocorticoids?
E Silva PM, Carvalho VF, Cordeiro RS, Martins MA
Neuroimmunomodulation 2008. Epub ahead of print.
|
|

|
Th17 cells in human disease
Tesmer LA, Lundy SK, Sarkar S, Fox DA
Immunol Rev 2008. 223:87-113
|
|

|
Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis
Ivory K, Chambers SJ, Pin C, Prieto E, Arques JL, Nicoletti C
Clin Exp Allergy 2008. 38(8):1282-1289
|
|

|
Novel immune regulatory pathways and their role in immune reconstitution syndrome in organ transplant recipients with invasive mycoses
Singh N
Eur J Clin Microbiol Infect Dis 2008. 27(6):403-408
|
|

|
In Vitro Differentiation of Dendritic Cells in the Presence of Prostaglandin E2 Alters the IL-12/IL-23 Balance and Promotes Differentiation of Th17 Cells
Khayrullina T, Yen JH, Jing H, Ganea D
J Immunol 2008. 181(1):721-735
|
|
|
Commentary on recent genetic studies
Rhodes D
Partn Seek Cure Newsl 2007. 3(1):12-15
|
|
|