Editorial
| Rev Diabet Stud,
2007,
4(3):126-133 |
DOI 10.1900/RDS.2007.4.126 |
DPP-4 Inhibitors and Combined Treatment in Type 2 Diabetes: Re-evaluation of Clinical Success and Safety
Harold W. de Valk
Department of Internal Medicine, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands, e-mail: H.W.devalk@umcutrecht.nl
Keywords: type 2 diabetes, incretin analogues, DPP-4 inhibitor, glycemic control, safety
Abstract
The incretin system has proven to be a new source of glucose-lowering drugs. Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) are the incretins which are degraded by dipeptidyl peptidase-4 (DPP-4). GLP-1 is the major relevant incretin in type 2 diabetes, GIP has little stimulatory capacity. Oral inhibitors of DPP-4 increase GLP-1 levels and this leads to lower glucose levels caused by increased insulin secretion and decreased glucagon levels. There are currently two oral drugs registered with the European Medicinal Evaluation Agency: sitagliptin and vidagliptin. Both compounds have shown similar effects to date. A main issue is to establish the value of this new class of drugs in the treatment of patients with type 2 diabetes. In this article, results from randomized studies on the efficacy of the new drugs are discussed: 1. comparison with placebo to establish long-term efficacy, 2. comparison with placebo when added to the regimen in patients failing on another oral glucose-lowering drug and 3. comparison in a head-to-head trial with other conventional drugs. Also, the combination with insulin is a promising new avenue. Both efficacy and safety (regarding hypoglycemia, body weight changes and changes in lipid levels) are major components in the decision of the optimal pharmacological treatment, which is discussed in this article. Finally, the advantages, disadvantages and risks of the new anti-diabetic compounds are highlighted, which are applicable to other classes of diabetes drugs.
Introduction
Considering the rapid global increase in the prevalence of type 2 diabetes and the difficulties experienced in achieving or sustaining adequate glycemic control with currently available drugs, there is a need to develop new drugs [1, 2]. Optimally, these drugs would combine the best long-term efficacy and safety profiles with the lowest possible risk of hypoglycemia. The incretin system has attracted much attention and recently a number of new drugs related to this system has become available [3-6].
The incretin system has been described in detail in a number of recent reviews. Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) are the two gut-derived peptide hormones involved in this system. The incretin system was described after it was shown that the insulin response after an oral glucose load was much stronger than that after a similar load given intravenously [7, 8]. This increased response is explained by the glucose-related release of GLP-1 and GIP from the gut. Both GLP-1 and GIP have very short half-lives and are metabolized by dipeptidyl peptidase-4 (DPP-4), a cell membrane-bound enzyme, also known as CD26 [4, 6]. After the incretin system was discovered, it became evident that this system could add new approaches to the treatment of patients with type 2 diabetes. Since GIP has little effect on insulin production in type 2 diabetes, the focus shifted to GLP-1 [2]. The development and application of GLP-1 analogues, such as exenatide and liruglatide, showed appropriate efficacy in the treatment of type 2 diabetes. However, for sufficient efficacy both compounds need to be injected subcutaneously. Since GLP-1 is metabolized by DPP-4, the next logical step was to search for inhibitors of this enzyme. Two compounds have so far been developed: sitagliptin and vildagliptin.
In order to apply this new class of glucose-lowering drugs properly in clinical practice, a number of questions need to be answered. Firstly, efficacy, degree of effect and dose-effect relation in patients with type 2 diabetes have to be demonstrated in long-term application. Secondly, effective combinations of the new drugs with other compounds have to be found. Finally, the relative efficacy of these compounds and combinations has to be compared with current classes of drugs and conventional therapies. In this paper, existing data on the efficacy and value of these compounds in clinical application as well as their safety are re-evaluated.
Efficacy of new generation DPP-4 inhibitors
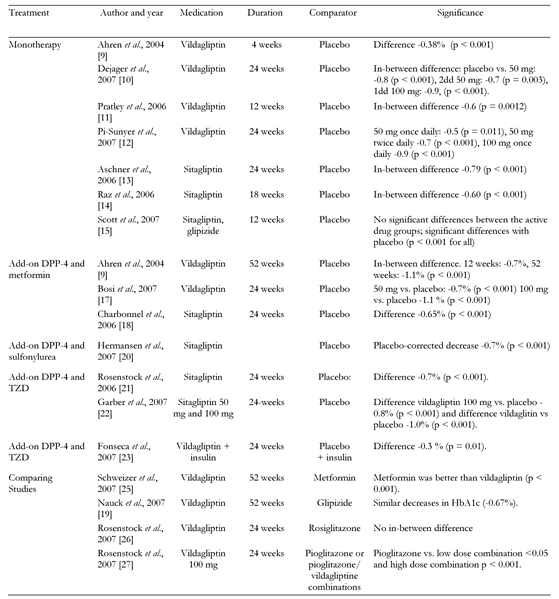
In order to find a satisfactory answer to the first question, the new compounds were used as initial medication in drug-naïve patients with type 2 diabetes and compared with placebo (Table 1). Ahren et al. used LAF237 (vildagliptin) in a 4-week, randomized, placebo-controlled study in diet-treated patients with type 2 diabetes. It was shown that DPP-4 inhibition resulted in statistically significant lower mean 24-hour glucose levels, lower fasting glucose levels, lower mean 4-hour post-breakfast glucose levels and lower peak post-breakfast glucose levels, without changes in fasting or mean 24-hour insulin levels [9]. A decrease in glucagon was related to improved glucose levels, whereas no such association was demonstrated for insulin. These data emphasized the importance of glucagon in relation to the glucose-lowering effect caused by DPP-4 inhibition. Interestingly, insulin levels were largely unchanged in LAF237-treated patients compared with patients who received placebo, while, at the same time, glucose levels were lower. This suggested that either insulin effectiveness was increased or insulin secretion was stimulated.
Table
1.
Characteristics of and effect on HbA1c observed in randomized clinical trials using DPP-4 inhibitors |
|
 |
 |
Different doses of vildagliptin were tested by Dejager et al. in a 24-week study and compared with placebo [10]. All doses of vildagliptin (50, 2dd 50 mg and 1dd 100 mg) were significantly better than placebo. There was no association between dose and response for the whole group. However, the positive dose-response was observed in patients with higher baseline HbA1c-levels. In another 12-week study comparing 50 mg vildagliptin with placebo in drug-naïve patients, the dose-response was similarly higher with higher baseline HbA1c-levels [11]. Pi-Sunyer et al. showed that, in drug-naïve patients, 50 mg vildagliptin once daily, 50 mg twice daily and vildagliptin 100 mg once daily all decreased HbA1c significantly compared with placebo [12]. A dose-effect relationship was evident in patients with HbA1c > 8.0%, but not with in those with HbA1c ≤ 8.0% at baseline.
Sitagliptin reduced HbA1c levels significantly compared with placebo in a study by Aschner et al. [13]. An indirect assessment of β-cell function using HOMA-B showed improved function. However, no data on glucagon were provided. A similar beneficial effect on glycemic control was observed with sitagliptin (100 mg or 200 mg daily) compared with placebo in another study [14]. Both sitagliptin and vildagliptin were well tolerated.
Finally, Scott et al. used an intricate, 6-group, randomized, placebo-controlled, parallel approach to study the effects of 4 different doses of sitagliptin and to verify its effect in comparison with glipizide [15]. All active drug groups showed a significant decrease in HbA1c compared with placebo with no significant differences between the groups. The authors concluded that 100 mg sitagliptin would be the best dose since the decrease with this dose was the greatest and most stable. These studies clearly show efficacy in long-term use in humans, which answers the first question.
Combination therapy with DPP-4 inhibitors and metformin
The second question considers the efficacy of the new drugs when added to conventional drug therapy where conventional therapy has failed. This is particularly relevant in type 2 diabetes, which is an inherently progressive disease because of gradually increasing β-cell failure.
Metformin
Vildagliptin or placebo was given as an add-on to patients on stable doses of metformin, who had an HbA1c between 7.0 and 9.5% (thus excluding the well-controlled patients), in a 52-week randomized trial [16]. After the first 12 weeks, HbA1c decreased by 0.6% on vildagliptin but increased by 0.1% on placebo [16]. The difference (-0.7%) was significant. After 52 weeks, the difference between HbA1c in the vildagliptin and the placebo group was even higher (-1.1%, p < 0.001). HbA1c on placebo continued to rise, while it remained stable in the vildagliptin group. Bosi et al. showed similar results in a 24-week randomized placebo-controlled trial comparing 50 mg and 100 mg vildagliptin as add-on to metformin in inadequately-controlled patients on metformin monotherapy [17]. Charbonnel et al. obtained comparable results for sitagliptin [18].
Another approach was to compare the effect of adding sulfonylurea or sitagliptin to patients failing on metformin [19]. It turned out that both strategies had an equal effect on HbA1c. All these studies demonstrate the good effect of DPP-4 inhibition when added to metformin in patients who were not optimally controlled, even though other combinations seem to be equally effective.
Sulfonylureas
No trials were carried out on pure add-on effects of DPP-4 inhibitors compared with placebo in patients who failed efficient glucose control on sulfonylurea. A study by Hermansen et al. [20] comes close to dealing with this question. The investigators applied supplementary therapy by sitagliptin to patients who failed on glimepiride and compared this regimen with placebo. After 24 weeks, the decrease of HbA1c levels in patients on sitaglitin compared to those on placebo was -0.57% (p < 0.001).
Thiazolidinediones
One study has addressed the effect of adding a DPP-4 inhibitor to a TZD (pioglitazone) [21]. Adding sitagliptine or placebo to pioglitazone treatment in patients with inadequate control on monotherapy with the TZD showed that the difference between HbA1c in the two groups was -0.7% (p < 0.001) in favor of vildagliptin-pioglitazone treatment. In another study, investigators added vildagliptin 50 mg, vildagliptin 100 mg or placebo to pioglitazone in patients failing on TZD [22]. The combined treatments with vildagliptin resulted in significant HbA1c decreases compared with placebo.
Insulin therapy
So far, only one study has been published on the combination of insulin with DPP-4 inhibitors [23]. In this study, it was shown that adding 50 mg vildagliptin to insulin therapy was associated with a significant decrease in HbA1c compared with placebo. This effect was observed in subjects older than 65 years but not in younger subjects. Another important observation was that significantly fewer severe hypoglycemic events occurred when vildagliptin was added to insulin instead of placebo, even though the general incidence of adverse effects was similar in both treatment groups. In the vildagliptin-insulin group, the event rate in confirmed hypoglycemia was 1.95 per event-year compared to 2.96 per event-year with placebo. In contrast, no severe hypoglycemia was observed with vildagliptin compared to 0.1 event per patient-year in the placebo group. Also, with vildagliptin, total cholesterol and LDL-cholesterol decreased significantly compared to placebo, whereas there were no significant differences in body weight change. These initial results demonstrated a potential major contribution of DPP-4 inhibition on the incidence of hypoglcyemia in patients with type 2 diabetes treated with insulin. It is possible that the effects of GLP-1 do not only suppress glucagon levels at times of hyperglycemia but also increase these levels at times of hypoglycemia [24]. In conclusion, adding DPP-4 inhibitors to patients failing on metformin or TZD monotherapy results in a reduction of HbA1c levels by 0.5-1.0% compared with placebo and this answers the second question satisfactorily.
Head-to-heat trials with DPP-4 inhibitors and conventional drug regimens
The third question refers to the efficacy of the new drugs (DPP-4 inhibitors) compared with established drug treatment regimens containing metformin, sulfonylureas and thiazolidinediones (TZD). This question is very important for clinical practice because we need to make the best choice between a number of glucose-lowering drug classes.
Schweitzer et al. looked at the effects of monotherapy with vildagliptin compared with those of monotherapy with metformin [25]. In a 52-week, randomized, parallel-group study design 100 mg vildagliptin (given in two doses) was compared with metformin. A significant decrease in HbA1c was observed in both groups, but the decrease was significantly greater in patients on metformin than in patients on vildagliptin. This difference was observed in patients with HbA1c > 8.0%, but not in patients with HbA1c ≤ 8.05 [25]. Approximately 75% of the patients in both groups completed the trial. Therefore, at present, there is no compelling reason to see DPP-4 inhibitors as the drug of first choice in type 2 diabetes.
Rosenstock et al. compared rosiglitazone monotherapy with vildagliptin monotherapy [26]. Vildagliptin showed an effect on HbA1c comparable to that of rosiglitazone. Both compounds were associated with greater decreases in patients with baseline HbA1c values >9.0%. Rosiglitazone was associated with significantly more weight gain as expected. There was only one mild hypoglycemic event in both groups and no severe hypoglycemic events. In both groups, approximately 85% of patients completed the study. Therefore, DPP-4 can be seen as equivalent to TZD regarding effects on glycemic profile and tolerability, while rosiglitazone is associated with more weight gain. Recently, Rosenstock et al. published another study with a more complicated design, which compared, in drug-naïve patients, four strategies over 24 weeks: vildagliptin 100 mg monotherapy, pioglitazone 30 mg monotherapy, vildagliptin-pioglitazone 50/15 mg and vildagliptine 100 mg/30 mg therapy [27]. The decreases in HbA1c per strategy were -1.1% for vildagliptine monotherapy, -1.4% for pioglitazone monotherapy, -1.7% for the low-dose combination therapy and -1.9% for the high-dose combination therapy. The combination therapies were both significantly better than pioglitazone monotherapy.
In conclusion, it cannot be regarded as proven to date that DPP-4 inhibitors are superior to the standard approach of metformin as first therapy and that it has an equally positive potential when comparing DPP-4 inhibitors with biguanides or TZDs.
Side effects
The incidence and nature of side effects are the third major question in choosing a specific class of drugs. Side effects to be considered are changes in body weight or lipid levels and incidence of hypoglycemia. In those studies that describe these parameters, there is no consistent major change in body weight or lipid parameters [9-12, 26, 16-18, 26, ]. The exceptions are that total and LDL-cholesterol were significantly lower on the vildagliptin-insulin combination compared with placebo-insulin [23] and that triglycerides were higher after metformin plus placebo compared with metformin plus vildagliptine [17]. As expected, rosiglitazone was associated with a higher body weight compared to vildagliptin [26]. In the same study, vildagliptin was associated with lower total and LDL-cholesterol and triglycerides, as well as lower HDL compared with rosiglitazone. With sitagliptin monotherapy, triglycerides were significantly lower than with placebo [15]. It should be noted that, in these studies, many patients did not use statins. Guidelines now recommend the the use of statins for many, if not all, patients with type 2 diabetes and it is questionable whether the observed effects reported here in statin-naïve patients persist if all patients are treated according to this guideline in clinical practice.
With regard to sulfonylurea, the expected weight increase was observed and was statistically significant when compared with placebo or sitagliptin in two studies [15, 19]. Comparing glipizide with sitagliptin as an add-on to metformin showed the expected increase in weight in the glipizide group, while weight decreased in the sitagliptin group. The difference in weight changes between the two treatment groups was significant [19]. Hypoglycemia was a rare event in these studies, even when DPP-4 was studied in combination with insulin therapy [10-12, 16, 18, 23, 26, 27].
The incidence of hypoglycemia was expectedly increased with sulfonylurea [15, 19]. In summary, side effects do not seem to be a major problem in the application of DPP-4 inhibitors. There is no change in body weight, which occurs on treatment with sulfonylurea derivatives or TZDs, and a potential beneficial effect on LDL and triglycerides.
Discussion
DPP-4 inhibitors are a new class of drugs that improve glycemic control by inhibiting glucagon secretion in states of hyperglycemia and stimulate insulin secretion in a glucose-dependent manner. Insulin sensitivity may also improve. Studies comparing DPP-4 inhibitors with placebo in drug-naïve patients with type 2 diabetes unequivocally demonstrate an improvement in glucose levels without a major increase in body weight or a high incidence of mild or severe hypoglycemia.
For many years, metformin has been the drug of choice in patients who need to start on oral medication. Metformin is effective, does not cause weight gain, involves little risk of hypoglycemia and is very cheap because it is out of patent. Due to these advantages, it is necessary to show that vildagliptin is non-inferior to metformin. Published data from DPP-4 inhibitor studies do not allow conclusive evaluation, but when data from studies comparing metformin with vildagliptin [17, 25] is taken together, it is evident that monotherapy could end somewhere equal or slightly inferior to metformin.
We can also retain that metformin works better for glycemic control than sulfonylurea derivatives such as glyburide. The ADOPT trial compared the effects of monotherapy with rosiglitazone (a TZD), metformin or glyburide on glycemic control in drug-naïve patients [28]. The least risk of failure detected with rosiglitazone monotherapy (15%) was significantly lower than with metformin (21%) and glyburide (34%), while metformin did significantly better than glyburide.
A comparison of different doses of sitagliptin and glipizide (sulfonylurea) monotherapy showed that the effects on glycemic control were roughly equal [19]. Sulfonylurea derivatives progressively lose their efficacy in the course of months to years. Sulfonylurea derivatives and TZDs cause an increase in body weight, although TZDs induce a favorable shift in fat distribution. DPP4-inhibitors do not cause weight gain or only to a very limited degree. In the light of this, there is a place for these drugs as monotherapy. Combining DPP4-inhibitors with other classes of oral glucose-lowering drugs can provide a powerful therapy. DPP-4 can be combined with drugs with a different mechanism of action like metformin and TZD.
Regarding the mechanism of DPP-4 inhibitor action, inhibition of glucagon release seems to be the major effect, with an adverse association between glucagon decrease and glucose level reduction. In addition to this mechanism, there are strong indications of a relative increase in insulin secretion and an increase in insulin sensitivity. Sulfonylurea derivatives increase insulin secretion by interfering with the potassium channels in pancreatic β-cells. Based on these considerations, fixed combination therapies of DPP4-inhibitors with other drug classes can be expected in the near future.
An intriguing possibility is the combination of DPP-4 inhibitor with insulin [23]. This combination is interesting, because the only study performed to date showed that glycemic control improved but without an increase in hypoglycemic events. There was even a lower incidence of severe hypoglycemia. For comparison, the ‘treat-to-target’ trial provides a strong argument for using long-acting insulin analogues instead of human long-acting insulin in patients with type 2 diabetes, who take a combination of long-acting insulin with oral medication [29]. However, long-acting analogue insulin was associated with fewer mild hypoglycemic events, but not with fewer severe hypoglycemic events. Although it should be noted that, in the study by Fonseca et al., more complex insulin therapy was used than in the ‘treat-to-target’ trial, these results still indicate the potential value of DPP-4 inhibitors in combination with insulin [23].
Recent publications on the adverse effects of TZD remind us of the potential long-term risks of new compounds, which are not readily detected in the development phase, during the initial period after the start of marketing and during widespread use. Meta-analysis of cardiovascular effects of rosiglitazone caused doubts about the safety of this compound [30]. In the aftermath of that publication, serious doubts on the quality of the specific meta-analysis emerged and editorials fought battles over the interpretation of this and other subsequent meta-analyses [31-34]. It also showed the weaknesses inherent in meta-analytic procedures. On top of that, an interim analysis of a trial focusing on the possibility of cardiovascular damage by rosiglitazone in the same journal as the firstly mentioned meta-analysis [30] showed no negative effects [35]. To date, no negative advice on TZD has been issued. This shows that utmost care with the prescription of drugs is as important as it has ever been. It is important to weigh the pros and cons carefully in each individual situation. And an open mind is also as important as ever. As David Nathan pointed out recently, the glucose-lowering market is exploding in the same way as the global epidemic. Economic interests are huge, especially because optimally effective and safe drugs have yet to be found, if they ever will be. As a consequence of the huge economic impact on the treatment of diabetes, the use of new medications is restricted by reimbursement policies that differ from country to country and that can prevent or slow down the introduction of innovative medicines.
 |
 |
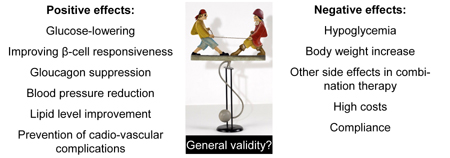
Figure 1. Conceptual model on advantages, disadvantages and risks of the new glucose-lowering drugs based on DPP-4 inhibition. However, it needs to be noted that positive and negative effects are detected in study sample populations. It is questionable if the model can be extrapolated to all type 2 diabetes patients. The optimal therapies for patients need to be verified in individual cases with innate clinical characteristics. |
|
Figure 1 puts the introduction and long-term use of glucose-lowering medication into a conceptual model. A major question is whether the results of a trial can be extrapolated to the specific patient in front of us. For example, do all the data of the UKPDS studies still apply to current medical practice? Next, the benefits and disadvantages need to be weighed against each other and the suggested long-term side effects of TZDs are an example of side effects unknown when these compounds were first marketed. The TZD story underscores that these considerations need to be applied to all other new drugs. Both metformin and sulfonylurea were temporarily banned, or nearly banned, because of side effects during their history. Therefore, DPP-4 inhibitors should be used with caution, in the hope that they will indeed fulfill their promise.
References
- Wild S, Rosglic C, Green A, Sicree R, King H. Global prevalence of diabetes; estimates for the year 2000 and projections for 2030. Diabetes Care 2004. 27:1047-1053. [DOD] [CrossRef]
- Nathan DM. Finding new treatments for diabetes – how many, how fast … how good? New Engl J Med 2007. 356:437-440. [DOD]
- Gallwitz B. New therapeutic strategies for the treatment of type 2 diabetes mellitus based on incretins. Rev Diabet Stud 2005. 2:61-69. [DOD] [CrossRef]
- Drucker D, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006. 368:1696-1705. [DOD] [CrossRef]
- Ahren B. Dipeptidyl peptidase-4 inhibitors. Clinical data and clinical implications. Diabetes Care 2007. 30:1344-1350. [DOD] [CrossRef]
- Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes. Diabetes Care 2007. 30:1335-1343. [DOD] [CrossRef]
- Creutzfeldt W. The incretin concept today. Diabetologia 1979. 16:75-85. [DOD] [CrossRef]
- Creutzfeldt W. Entero-insular axis and diabetes mellitus. Horm Metab Res Suppl 1992. 26:13-18. [DOD]
- Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004. 89:2078-2084. [DOD] [CrossRef]
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007. 39(3):218-223. [DOD] [CrossRef]
- Pratley RE, Jauffret-Kramel S, Galbreath E, Holmes D. Twelve-week monotherapy with DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res 2006. 38:423-428. [DOD] [CrossRef]
- Pi-Sunyer FX, Schweitzer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diab Res Clin Pract 2007. 76:132-138. [DOD] [CrossRef]
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006. 29(12):2632-2637. [DOD] [CrossRef]
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes. Diabetologia 2006. 49:2564-2571. [DOD] [CrossRef]
- Scott R, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients wth type 2 diabetes. Clin Pract 2007. 61:171-180. [DOD] [CrossRef]
- Ahren B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF 237 in metformin-treated patients with type 2 diabetes. Diabetes Care 2004. 27:2874-2880. [DOD] [CrossRef]
- Bosi E, Camisaca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007. 30:890-895. [DOD] [CrossRef]
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006. 29:2638-2643. [DOD] [CrossRef]
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007. 9:194-205. [DOD] [CrossRef]
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitud inadequately controlled on glimepiride alone or glimepiride and metformin. Diabetes Obes Metab 2007. 9:733-745. [DOD] [CrossRef]
- Rosenstock J, Brazg R, Andryuk PJ, Lu Km Stein P, Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitragliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: A 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006. 10:1556-1568. [DOD] [CrossRef]
- Garber AJ, Schweitzer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007. 9:166-174. [DOD] [CrossRef]
- Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007. 50:1148-1155. [DOD] [CrossRef]
- Dunning BE, Foley JE, Ahren B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 2005. 48:1700-1713. [DOD] [CrossRef]
- Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA1c over 1 year in drug-naïve patients with type 2 diabetes. Diabet Med 2007. 24:955-961. [DOD] [CrossRef]
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes. Diabetes Care 2007. 30:217-223. [DOD] [CrossRef]
- Rosenstock J, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazon compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2007. 29:175-185. [DOD] [CrossRef]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G, ADOPT Study Group. Glycemic durability of rosiglitazone, metformin or glyburide monotherapy. New Engl J Med 2006. 355(23):2427-2443. [DOD] [CrossRef]
- Riddel MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The Treat-To-Target trial. Randomized addition of glargine of human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003. 26:3080-3086. [DOD] [CrossRef]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. New Engl J Med 2007. 356(24):2457-2471. [DOD] [CrossRef]
- Psaty BM, Furberg CD. Rosiglitazone and cardiovascular risk. New Engl J Med 2007. 356(24):2522-2524. [DOD] [CrossRef]
- Rosiglitazone: seeking a balanced perspective. Lancet 2007. 369(9576):1834. [DOD]
- Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 2007. 289(10):1189-1195. [DOD] [CrossRef]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007. 298(10):1180-1188. [DOD] [CrossRef]
- Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ, RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes - an interim analysis. New Engl J Med 2007. 357(1):28-38. [DOD] [CrossRef]
This article has been cited by other articles:
|