Review
| Rev Diabet Stud,
2011,
8(3):432-440 |
DOI 10.1900/RDS.2011.8.432 |
Metabolic Memory and Individual Treatment Aims in Type 2 Diabetes – Outcome-Lessons Learned from Large Clinical Trials
Cristina Bianchi, Stefano Del Prato
Department of Endocrinology and Metabolism, Section of Diabetes and Metabolic Diseases, University of Pisa, Italy
Address correspondence to: Stefano Del Prato, e-mail: stefano.delprato@med.unipi.it
Manuscript submitted October 26, 2011; accepted October 30, 2011.
Keywords: antihyperglycemic therapy, cardiovascular risk, glycemic control, glycemic legacy, macrovascular, microvascular complication, type 2 diabetes, UKPDS
Abstract
Reducing the burden of long-term complications in type 2 diabetic patients remains a major task, and represents a huge challenge. Whilst tight glycemic control has been shown to reduce the risk of microvascular complications, controversy remains regarding the benefit of intensive treatment in relation to the prevention of cardiovascular events. Recent large trials (including ACCORD, ADVANCE, and VADT) were unable to show a significant impact of glycemic control on cardiovascular events. Also, it has been argued that these trials included patients with a long duration of the disease, and with previous unsatisfactory glycemic control. Chronic exposure to hyperglycemia may cause a kind of negative metabolic memory, and thereby reduce the potential impact of good glycemic control. This concept has been corroborated by the UKPDS which recruited only subjects with newly diagnosed diabetes and without prior cardiovascular events. In these patients, early achievement of glycemic control translated into a long-term reduction of the risk of micro- and macrovascular complications. This observation prompted the UKPDS investigators to propose a positive "glycemic legacy", supporting the need for early and appropriate treatment of hyperglycemia and associated metabolic disturbances. This should be feasible now through the selection of individual targets and personalized pharmacologic treatments. In doing so, the potential risks of intensive treatment might then be avoided.
Abbreviations: ABCD - age, body weight, complications, diabetes duration; ACCORD - Action to Control Cardiovascular Risk in Diabetes (trial); ADA - American Diabetes Association; ADVANCE - Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; AGE - advanced glycation end-product; AHA - American Heart Association; CI - confidence interval; CV - cardiovascular; EASD - European Association for the Study of Diabetes; FPG - fasting plasma glucose; HbA1c - glycated hemoglobin A1c; HR - hazard ratio; LDL - low-density lipoprotein; PKC - protein kinase C; RR - relative risk; T2D - type 2 diabetes; UKPDS - UK Prospective Diabetes Study; VADT - Veteran Affairs Diabetes Trial
Introduction
A recent analysis published by Danaei et al. has revealed an even more dramatic picture of the ongoing “epidemic” of diabetes across the world than was once foreseen [1]. In the 10 world regions examined, the prevalence of diabetes has been steadily increasing in both genders during the period 1980-2008. The overall figure shows that in 1980, the global diabetic population was 153 million. This figure has more than doubled since 2008, reaching the extraordinary number of 357 million. If growth continues at the same rate, then future humanity will be facing an even greater societal and economical problem than at present. The conclusion of the paper by Danaei et al. was very straightforward. The authors observed that "effective preventive interventions are needed, and health systems should prepare to detect and manage diabetes and its sequelae" [1]. Indeed, the major burden of diabetes originates from the elevated risk of its dreadful complications, and its sequelae. Among people with diabetes, the prevalence of complications remains unacceptably high. Deshpande et al. have recently reported that up to 30% of the diabetic population have some microvascular complications, and at least 10% already have had a cardiovascular (CV) event [2]. Applying these proportions to the figures from Danai et al., we can estimate that approximately 110 million diabetic patients will have microvascular complications, and 40 million will experience CV events.
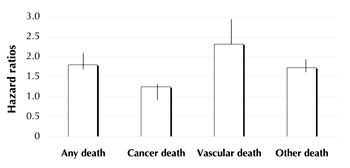
Multiple factors contribute to CV risk in diabetes. However, hyperglycemia, the hallmark of the disease, constitutes a powerful capacity to predict mortality even in the general population. The Emerging Risk Factors Collaboration has clearly indicated how increased plasma glucose levels are associated with a significant increase in the risk of mortality in different diseases, including cancer and vascular disease, even in the non-diabetes population [3] (Figure 1). The relationship is so strong that risk increases with the elevation of plasma glucose in an almost linear fashion. When looking at this relationship, one could argue that lowering plasma glucose levels towards the normal range should be associated with reduction of risk for morbidity and mortality of all causes. Although this concept appears intuitive, conclusive proof does not emerge from the results of the most recent intervention trials.
 |
 |
Figure 1. Hazard ratios for major causes of death. Diabetes vs. non-diabetes [1]. |
|
Intervention trials in diabetes
The United Kingdom Prospective Diabetes Study (UKPDS) was the first trial to provide strong evidence that appropriate glycemic control could lead to a significant reduction of the risk for long-term diabetic complications [4]. The trial recruited 3,867 newly diagnosed type 2 diabetes (T2D) patients who were randomly assigned to intensive treatment with a sulfonylurea or insulin, or to conventional management, mainly based on diet [4]. Over the 10-year follow-up, average hemoglobin A1c (HbA1c) was 7.0% in the intensive-treatment group compared with 7.9% in the conventional group. Compared with the latter, the risk of developing diabetes complications in the intensive-treatment group was reduced by 12% (95% confidence interval (CI) 1-21, p = 0.029) for any diabetes-related endpoint, 10% (95% CI 11-27, p = 0.34) for any diabetes-related death, and 6% (95% CI 10-20, p = 0.44) for all causes of mortality. In the diabetes-related aggregate endpoint, the risk reduction in microvascular complications amounted to 25% (95% CI 7-40, p = 0.0099). Also, a 16% reduction in the risk of myocardial infarction was reported, although this was only close to statistical significance (p = 0.052). This finding led to much discussion, and left the question unresolved whether glycemic control may contribute to a reduction of the CV risk in diabetes.
The issue was not solved by the results in the Kumamoto study [5]. In this trial, a small number of Japanese patients on intensive insulin treatment achieved much better glycemic control (HbA1c 7.1% vs. 9.45%) than those on conventional insulin therapy. In the intensively treated patients, the cumulative percentages of the development and progression in retinopathy, nephropathy, and neuropathy were significantly lower. After 8-year follow-up, there was also an apparent positive effect on macrovascular complication, as indicated by an almost 50% reduction in the number of CV events in intensively vs. conventionally treated subjects. Unfortunately, the absolute number of events was too small to allow formal statistical analysis, so that no final conclusion could be drawn.
Five thousand and thirty-eight T2D patients with evidence of macrovascular disease were recruited in the PROactive trial [6]. The patients were randomly assigned to receiving oral pioglitazone, or placebo, added to any existing glucose-lowering medication. During the 34.5-months of observation, there was no significant reduction in the primary CV endpoint with pioglitazone (hazard ratio (HR) 0.90, 95% CI 0.80-1.02, p = 0.095). Whereas, a statistical significance was achieved for the pre-defined secondary endpoint, i.e. a composite of all-cause mortality, non-fatal myocardial infarction, and stroke (HR 0.84, 95% CI 0.72-0.98, p = 0.027). In summary, the PROactive trial could not prove beyond all reasonable doubt, that intensive glycemic control provides a solid benefit with respect to prevention, or reduction, in CV risk in T2D patients.
More recently, the results of three large intervention trials [7-9], enrolling a total of 23,000 T2D patients, revived the debate on the relationship between glycemic control and CV outcomes. In the ADVANCE study (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation), a lower mean HbA1c level was achieved in the intensive-control group than in the standard-control group (6.6 vs. 7.3%) [7]. Intensive control reduced the incidence of combined major macro- and microvascular events (HR 0.90, 95% CI 0.82-0.98, p = 0.01), and major microvascular events (HR 0.86, 95% CI 0.77-0.97, p = 0.01). In contrast, there was no significant effect from glucose control on major macrovascular events, death from CV causes, or death from any cause. In the Veteran Administration Diabetes Trial (VADT) [8], median HbA1c levels were 8.4% in the standard-therapy group, and 6.9% in the intensive-therapy group. There was no significant difference between the two groups in the rate of CV events, or in the rate of death from any cause (HR 1.07, 95% CI 0.81-1.42, p = 0.62). Likewise, no differences between the two groups were observed for microvascular complications, with the exception of reduced progression of diabetic nephropathy. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [9] was prematurely discontinued because of a 22% increased in risk mortality (95% CI 1.01-1.46) in the intensively treated group.
Interpreting the results of large clinical trials
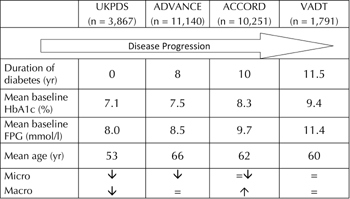
Although the results of the recent large clinical trials sound clear-cut, it is worth critically analyzing their features [10]. The VADT, ACCORD, and ADVANCE studies included individuals at high CV risk. This is apparent from the high prevalence of patients with prior CV events (35%), and more than 50% having microvascular complications [8-10]. Because of the high CV risk, an aggressive treatment of CV risk factors was introduced to lower LDL-cholesterol (≈2.3 mmol/l) and blood pressure (≈120/70 mmHg). Also, anti-platelet therapy was used in 62-93% of the patients, and the number of people who were still smoking by the end of the study (8-17%) was reduced. Multifactor intervention has been already shown to be effective. Therefore, it is not surprising that the mortality rate (≈2.2% per year) in the trials was as low as in the general population. Under these conditions, it is difficult to demonstrate the benefits of tight glycemic control.
On the other hand, when patients without a prior CV event were evaluated, tight glycemic control was associated with a significant reduction of primary CV outcomes. A similar reduction could be observed in patients with HbA1c ≤ 8.0% at study entry, as compared with those with values ≥ 8.0%. One may assume that the lack of prior CV events, or microvascular complications, and a lower baseline HbA1c may reflect a shorter duration of the disease, and an overall better health status. Thus, duration of diabetes and prior CV events may be the key factors influencing the results of these recent trials, where strict glycemic control was achieved only after years of uncontrolled diabetes [10]. Ideal conditions for good glycemic control and health status prevail when diagnosis is made early and glycemic control is ensured from the time of diagnosis.
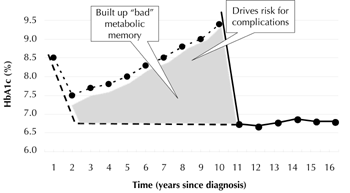
The difference between the ideal approach and what happens in the trials is graphically illustrated in Figure 2. It can easily be seen how this difference can i) lead to the development of diabetic complications, or ii) generate a "bad glycemic legacy". The latter relates to the "legacy effect", which was proposed from the post-trial results of UKPDS [11]; intensive treatment implemented at the time of diagnosis results in a sustained reduction in the risk of micro- and macrovascular complications. In the 10-year post-trial follow-up, patients originally randomized to intensive treatment maintained significant reductions in the rates of diabetes-related endpoints and microvascular complications. Also, they had a significant reduction in the risk of myocardial infarction (relative risk (RR) reduction of 15%, p = 0.0014) and all-cause mortality (RR reduction 13%, p = 0.007) [11]. These results were obtained although there were no longer differences in HbA1c values between patients originally assigned to conventional treatment, and those of the intensive-treatment group. Therefore, it was concluded that the legacy of good glycemic control in the initial stages of T2D translated into a permanent benefit related to micro- and macrovascular risk factors.
 |
 |
Figure 2. Hypothetical representation of the natural history of diabetic patients recruited in the Veteran Administration Diabetes Trial (VADT). The upper dotted line represents the HbA1c levels over time estimated on the basis of the average glucose profile described in the UKPDS. The lower dotted line represents the ideal time course of glycemic control. The solid line represents the time course of HbA1c in the VADT. Reprinted with permission from [9]. |
|
The relationship between diabetes duration before initiating intensive treatment and outcome is illustrated in Figure 3. The longer the duration, the smaller is the effect of tight glycemic control on diabetic complication. This view should lead to a change in the treatment of T2D, starting with implementation of appropriate treatment at the time of diagnosis, and leading to a reduction in treatment-associated risk for those patients with long disease duration. Early intervention is safer, and more effective, because of the probability of diabetic complications at diagnosis being relatively low. In this case, the "glycemic legacy" is of short duration, and is easier to modify. In these patients, targeting normoglycemia is feasible and necessary. In all cases, an uncompromised therapeutic approach should be applied, including the treatment of all CV risk factors.
 |
 |
Figure 3. Patient characteristics in large clinical diabetes trials. Compared with the pivotal United Kingdom Prospective Diabetes Study (UKPDS), which enrolled newly diagnosed patients, recent trials have enrolled high-risk patient populations characterized by a longer duration of disease, older age, and more severe hyperglycemia (i.e. higher HbA1c levels) at baseline [5-7]. |
|
The results from the extended phase of the STENO 2 trial provide compelling evidence that effective management of hyperglycemia, elevated blood pressure, and lipid disorders has beneficial health effects [12]. The study showed that, despite the lack of significant differences in cardio-metabolic risk factors, including HbA1c, systolic and diastolic pressure, triglyceride, total cholesterol and LDL cholesterol levels, a substantial difference in the incidence of defined endpoints was maintained over many years. The outcomes were much better in the intensive treatment group. These findings support the positive role of a "metabolic legacy", rather than simply a "glycemic legacy".
In summary, the UKPDS and STENO 2 studies provided evidence that intensive treatment of chronic hyperglycemia, and related metabolic abnormalities, in early stages of the disease, yield beneficial outcomes with long term effect [11, 12]. In contrast, a delay in effective treatment of metabolic disturbances can cause a spectrum of adverse biological reactions in vascular endothelial cells that may become irreversible. Preliminary work in endothelial cells has shown that hyperglycemia can induce changes in gene expression depending on modifications of histone tails (for instance, methylation). These changes persist, even after restoration of normoglycemia [13]. How these modifications persist over time is not clear. Epigenetic changes and biochemical processes (for example, advanced glycation) may contribute to the phenomenon, most likely as a consequence of sustained oxidative stress [14-16]. Excessive occurrence of free radicals triggers multiple intracellular pathways, including the activation of protein kinase C (PKC), increased fluxes through the polyol and hexamine pathways, and increased advanced glycation end-product (AGE) formation. Free radicals can also affect the expression of a number of genes involved in the pathogenesis of chronic diabetic complications [17-19]. Early and effective intervention can prevent the activation of this sequence of events. More importantly, it can prevent irreversible damages to molecular mechanisms involved in the pathogenesis of diabetic complications. Understanding the molecular events that enable prior glycemic control to result in end-organ protection in diabetes could lead to the development of new approaches for reducing the burden of diabetic complications.
In summary, tight glycemic control can exert a protective effect to prevent or minimize microvascular complications. However, for a beneficial effect on CV risk, intensive glycemic control needs to be implemented as soon as possible after the diagnosis of diabetes. This is an ambitious goal that requires appropriate intensive treatment. On the other hand, intensive glycemic control is challenging as it may inflict some undesired risks such as frequent hypoglycemia and increased mortality.
Phenotyping patients to reduce the risk
A more recent post-hoc analysis of the ACCORD study concluded that intensive therapy delayed the onset of albuminuria. Also, some measures of eye complications and neuropathy suggested a potentially positive effect of glycemic control on microvascular complications [20]. However, the investigators suggested to weigh the advantages against the risks, including increase in total and CV related mortality, increased weight gain, and risk for severe hypoglycemia. The trial was stopped earlier than planned because of a markedly increased death rate, with 52 more deaths among patients in the intensive treatment cohort.
The consideration that intensive treatment may be afflicted with severe risks seemed to be supported by recent data from Currie and co-workers [21]. The authors assessed the survival rate using the decile rank of HbA1c values in 27,965 T2D patients whose treatment was intensified from oral monotherapy to combination therapy with oral blood-glucose lowering agents. Also 20,005 patients were analyzed, whose treatment regimes were changed to include insulin [21]. The analysis confirmed the association between high HbA1c values and all-cause mortality and cardiac events. Also, it highlighted a similar association for low HbA1c values (<7.5%). However, some caution should be applied in interpreting these results. First of all, this was not an intervention randomized, placebo-controlled study, but rather a retrospective analysis with all the accompanying caveats. Moreover, a close look at the study cohorts reveals some interesting aspects. For instance, patients who switched from monotherapy to combined antihyperglycemic therapy showed an inverse relationship between age and HbA1c; the lower the HbA1c, the older the patients. Finally, the percentage of people with increased serum creatinine levels (>130 µmol/l) was higher in those with lower HbA1c values, suggesting a more severe impairment in kidney function. Interestingly, both age and glomerular filtration rate are independent predictors of CV mortality. The reasons for these associations are not readily apparent, but one may argue that elderly people with impaired renal function may be highly vulnerable. Intensive antihyperglycemic treatment may expose these patients to unwanted risks.
The concept of the "vulnerable T2D patient" has been supported by a recent post-hoc analysis of the ACCORD trial, showing that the relationship between average HbA1c and mortality differed between treatment strategies [22]. With intensive treatment, the risk of death increased continuously from an average HbA1c of 6.0% to that of 9.0%. Whereas, the curve for the standard strategy was distinctly nonlinear [22]. The excessive risk associated with intensive glycemic control occurred among those participants whose average HbA1c was >7% and did not change during active treatment. In these patients, treatment may have become more risky. In this regard, it is necessary to note that, in the ACCORD study, the risk of hypoglycemia was directly related with HbA1c levels: the smaller the response on glycemic control, the more aggressive was the treatment, and therefore the greater the risk of hypoglycemia. This risk may become dramatic in elderly patients, with co-morbidities (and multiple pharmacologic treatments) and impaired kidney function.
In recent trials, hypoglycemia was more prevalent in intensively treated patients [7-9]. Although not definitely proven in the trials, hypoglycemia may be a triggering factor of CV events in "vulnerable patients". A recent analysis of the ACCORD data has clearly indicated that the mortality rate is higher in those with hypoglycemia, regardless of the intensity of treatment [23]. However, in those with hypoglycemia, the mortality rate was obviously lower in people with tight glycemic control, as opposed to those with a lax glycemic control.
Similar results have been found in the ADVANCE study [24]. During follow-up, severe hypoglycemia was associated with a significant increase in adjusted risk rates of major microvascular and macrovascular events, as well as CV death or death from any cause (p < 0.001 for all comparisons). However, among patients reporting severe hypoglycemia, annual death rates were lower in the group receiving intensive treatment than in the group receiving standard treatment (3.6 vs. 5.1%). Also, hypoglycemia was associated with a range of non-vascular outcomes, including respiratory, digestive, and skin conditions (p < 0.01 for all comparisons).
In summary, although severe hypoglycemia may contribute to adverse outcomes, it is also possible that it is a sensitive marker identifying more vulnerable subjects. On a practical ground, reducing the risk of hypoglycemia appears to be important. A way to reduce it is to identify patients at increased risk, i.e. the most vulnerable patients. It was again the ACCORD study to suggest how to identify those subjects, as the risk of hypoglycemia was greater in patients with impaired renal function, with longer duration of diabetes, and in the older patients [25].
Intensive glycemic control is often associated with an increase in body weight. In the ACCORD trial, more than 25% of the intensively treated patients gained 10 kg over the study period, while in the VADT the average weight gain was >8 kg [9]. In the UKPDS, an average 5 kg body weight gain was recorded. However, intensive treatment did not prevent a significant improvement in microvascular complications, with an almost significant reduction in the risk of myocardial infarction [4]. The impact of increasing body weight during intensive treatment on CV outcome remains unclear, and should be a matter of further investigation.
Individualizing treatment aims
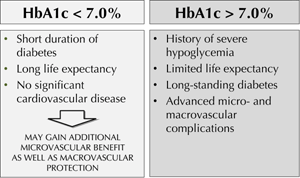
Based on the previous discussion, positive and negative effects of intensive treatment should be carefully considered in each case. The same conclusion is made by ADA and the American Heart Association (AHA). In a joint statement, these organizations invited physicians to identify different HbA1c targets for different diabetic subjects (Figure 4) [26]. Since the vast majority of patients enrolled in the intervention trials had a long duration of the disease, and a large proportion already had long-term complications, they advised that in these patients, and in those with limited life expectancy and history of severe hypoglycemia, the target HbA1c should be >7% [26]. In contrast, tighter glycemic control should be achieved and maintained (with HbA1c < 7%) in persons with short duration of diabetes, long life expectancy, no significant CV disease, and absence or presence of modest signs of microvascular complications. In these individuals, tight glycemic control may provide additional microvascular benefit.
 |
 |
Figure 4. Treatment goal personalization. Recommedations according to the American Diabetes Association and the American Heart Association [19]. |
|
Preventing the development of microangiopathic complications can also contribute to reduced CV risk. Both micro- and macrovascular complications share common pathogenetic defects such as oxidative stress. Moreover, microangiopathy is a systemic process involving all tissues of the body including the microvasculature of the heart. Such an involvement can contribute to an impaired outcome of atherogenic processes at the level of the coronary arteries, and contribute to the effect of traditional CV risk factors. In accordance with this hypothesis, diabetic retinopathy and other microvascular complications have been shown to be strong predictors of CV events [27].
Balancing risk and benefit of tight glycemic control
Glycemic control is recommended, but the expected benefits should be balanced against the potential risks which are associated with progressive but unsuccessful treatment intensity, such as severe hypoglycemia and body weight gain. In other words, the risk-to-benefit ratio must be determined individually, for each patient. This approach can only be processed by personalization of treatment goals and customized pharmacologic therapies.
Personalizing treatment may be rational, but it is not always a simple task, because concordant guidelines are lacking and physicians are not experienced with this method. A number of guidelines are available, but they tend to restrict rather than engage therapeutic options. To provide a user-friendly guideline for a personalized therapeutic approach for T2D patients, an independent university symposium was held at the EASD conference in Vienna, 2009. On this occasion, some elements were identified that may help to guide treatment selection. Also, the "A1C and ABCD of glycemia management in T2D" was proposed [28]. This method allows the individualization of the glycemic target based on age (A), body weight (B), complications (C), and duration of diabetes (D).
Age can be arbitrarily categorized as young (below 40), middle age, and elderly (>70). Individualized glycemic target and the speed of attainment of those targets can be selected based on this simple categorization (Figure 5). Body weight may help to guide initial pharmacologic intervention as body weight may reflect pronounced insulin resistance and differential CV risk profile. Complications should be evaluated in terms of increased CV and hypoglycemia risk and regarding treatment selection. Duration is likely to be linearly associated with the presence of co-morbidities and complications; it will require accurate fine-tuning in treatment to reduce the risk of severe hypoglycemia. In other words, drug selection and the HbA1c target should reflect the clinical status of the individual. Therefore, it is recommended that the pharmacological treatment in patients prone to hypoglycemia is carefully evaluated.
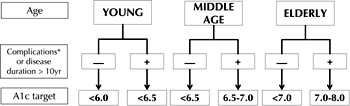 |
 |
Figure 5. Schematic representation of the HbA1c and ABCD strategies for recently diagnosed patients with type 2 diabetes. * Micro- and macrovascular complications. Adapted from [21]. |
|
Most recently, Ismail-Beigi and coworkers proposed a more comprehensive view for the individualization of glycemic targets in T2D [29]. Choosing a specific HbA1c target range for a given patient requires that several factors are taken into consideration. These include an assessment of the patient's risk for hyperglycemia-related complications versus the risks of therapy, co-morbid conditions, psychological status, capacity for self-care, economic considerations, family and social support systems.
Conclusions
We are convinced that the best interpretation of the recent intervention trials has been provided by one of the VADT principal investigators, who stated in the press conference: "If you go into a population that already has multiple risk factors, or prior CV disease, and long standing poor glucose control, you cannot expect benefits from glucose control in the short term. You can't expect miracles!" Poor metabolic control leads to the development of chronic diabetic complications, while good glycemic control at an early stage of diabetes may augment the patients' chance for a significant reduction of micro- and macrovascular risk. The metabolic memory may extend this beneficial effect over many years. Therefore, early and effective intervention is strongly recommended.
Early intervention with intensive treatment and consideration of individualized risk profiles is quite an ambitious goal. It is not easy to realize in practice until the therapeutic necessity is recognized and appropriate guidelines for individual treatment are available. To apply individual treatment effectively, the heterogeneity of type 2 diabetes must be recognized. Such heterogeneity is easy to keep in mind by just adding an "E" for etiology to the ABCD rule. The relative role of insulin resistance and beta-cell function must be appreciated to design pathophysiologic driven therapy. These could result in a "rule of thumb" or, even better, the five-finger rule (Figure 6). This rule together with the patient’s social-economic background could guide the physician to a more appropriate selection of glycemic targets and a more effective treatment for individual patients.
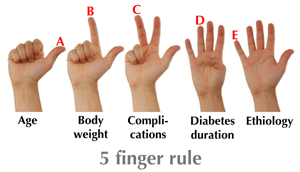 |
 |
Figure 6. The 5 finger rule for glycemic target personalization. |
|
Disclosures: SDP is member of the advisory panels of Novartis, Merck, Roche, Eli Lilly, Boehringer Ingelheim, Bristol-Myers Squibb, Astra Zeneca, GlaxoSmithKline, Sanofi, Takeda, and Novo Nordisk. He received research support from Merck, Sanofi, and Takeda.
References
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011. 378:31-40. [DOD] [CrossRef]
- Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther 2008. 88:1254-1264. [DOD] [CrossRef]
- The Emerging Risk Factors Collaboration Diabetes Mellitus, Fasting Glucose, and Risk of Cause-Specific Death. N Engl J Med 2011. 364:829-841. [DOD] [CrossRef]
- Intensive blood glucose control with sulfonylureas or insulin compared with conventional treatment and risk for complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998. 352:837-853. [DOD] [CrossRef]
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2003. 23:B21-B29. [DOD]
- Dormandy JA, Charbonnel B, Eckland DJ. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet 2005. 366:1279-1289. [DOD] [CrossRef]
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008. 358(24):2560-2572. [DOD] [CrossRef]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009. 360(2):129-139. [DOD] [CrossRef]
- ACCORD Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008. 358(24):2545-2559. [DOD] [CrossRef]
- Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia 2009. 52:1219-1226. [DOD]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008. 359:1577-1589. [DOD] [CrossRef]
- Gaede P, Lund Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008. 358(6):580 591. [DOD]
- Cooper ME. Metabolic memory: implications for diabetic vascular complications. Pediatr Diabetes 2009. 10:343-346. [DOD] [CrossRef]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001. 414:813-820. [DOD]
- Brownlee M. The pathobiology of diabetes complications: an unifying mechanism. Diabetes 2005. 54:1615-1625. [DOD]
- Ihnat MA, Thorpe JE, Kamat CD, Szabo C, Green DE, Warnke LA, Lacza Z, Cselenyak A, Ross K, Shakir S, et al. Reactive oxygen species mediate a cellular 'memory' of high glucose stress signalling. Diabetologia 2007. 50(7):1523-1531. [DOD]
- Wu WS, Tsai RK, Chang CH, Wang S, Wu JR, Chang YX. Reactive oxygen species mediated sustained activation of protein kinase C alpha and extracellular signal regulated kinase for migration of human hepatoma cell Hepg2. Mol Cancer Res 2006. 4(10):747-758. [DOD]
- Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol 2003. 1:95-108. [DOD] [CrossRef]
- Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol protein kinase C pathway in diabetes and hypergalactosemia. Diabetes 1994. 9:1122-1129. [DOD]
- Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010. 376(9739):419-430. [DOD] [CrossRef]
- Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010. 375:481-489. [DOD] [CrossRef]
- Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER. Action to Control Cardiovascular Risk in Diabetes Investigators Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010. 33:983-990. [DOD] [CrossRef]
- Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010. 340:B4909. [DOD] [CrossRef]
- Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010. 363:1410-1418. [DOD] [CrossRef]
- Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycatedhaemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010. 340:B5444. [DOD] [CrossRef]
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, et al. Intensive glycemc control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol 2009. 53(3):298-304. [DOD] [CrossRef]
- Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007. 30:292-299. [DOD] [CrossRef]
- Pozzilli P, Leslie RD, Chan J, De Fronzo R, Monnier L, Raz I, Del Prato S. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician’s personalized approach. Diabetes Metab Res Rev 2010. 26:239-244. [DOD] [CrossRef]
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011. 154:554-559. [DOD]
This article has been cited by other articles:

|
Efficacy and safety of repaglinide/metformin fixed-dose combination tablet in the treatment of type 2 diabetic patients
Wang X, Chen Q, Lyu X, Ye S, Jiang Z, Ni H, Wang W, Li K, Tang X, Yang J, Zhang X, Yu J, Zhang L, Zhang X, Zhong L, Li Q, Yang W
Chin J Endocrinol Metab 2014. 30(11):954-959
|
|

|
Insulin or surgery?
Blüher M
Chirurg 2014. 85(11):957-962
|
|

|
Differential transcriptional and posttranslational transcription factor 7-like regulation among nondiabetic individuals and type 2 diabetic patients
Pradas-Juni M, Nicod N, Fernandez-Rebollo E, Gomis R
Mol Endocrinol 2014. 28(9):1558-1570
|
|
|
The Role of MicroRNAs in Diabetic Complications - Special Emphasis on Wound Healing
Moura J, Borsheim E, Carvalho E
Genes (Basel) 2014. 5(4):926-956
|
|

|
Glycemic exposure, glycemic control, and metabolic karma in diabetic complications
Thomas MC
Adv Chronic Kidney Dis 2014. 21(3):311-317
|
|
|
Association among subclinical hypothyroidism, TSH levels and microvascular complications in Type 2 diabetic patients
Gao F, Poulle NP, Zafar MI, Shafqat RA
IOSR J Dent Med Sci 2014. 13(3):1-6
|
|

|
10-year incidence of diabetes and associated risk factors in Greece: the ATTICA study (2002-2012)
Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Pitaraki E, Metaxa V, Stefanadis C, ATTICA Study Group
Rev Diabet Stud 2014. 11(2):181-189
|
|
|
Impact of bariatric surgeries on diabetes outcomes
Muscelli E, Muscelli Alecrim H
Rev Soc Bras Clin Med 2014. 12(2):1-11
|
|

|
Antiglycation Activity of Iridoids and Their Food Sources
West BJ, Uwaya A, Isami F, Deng S, Nakajima S, Jensen CJ
Int J Food Sci 2014. 2014:276950
|
|

|
Role of metabolic surgery in less obese or non-obese subjects with type 2 diabetes: influence over cardiovascular events
Cohen R, Caravatto PP, Petry T, Cummings D
Curr Atheroscler Rep 2013. 15(10):355
|
|

|
The continuing need for drug development and clinical trials in type 2 diabetes and its complications: introduction to the RDS Special Issue
Raz I, Gallwitz B
Rev Diabet Stud 2011. 8(3):288-292
|
|
|