Original Data
| Rev Diabet Stud,
2004,
1(3):122-128 |
DOI 10.1900/RDS.2004.1.122 |
Visceral Obesity and Hemostatic Profile in Patients with Type 2 Diabetes: The Effect of Gender and Metabolic Compensation
Elzbieta Kozek, Barbara Katra, Maciej Malecki, Jacek Sieradzki
Department of Metabolic Diseases, Jagiellonian University, Medical College, 15 Kopernika Street, 31-501 Krakow, Poland.
Address correspondence to: Elzbieta Kozek, e-mail: ela_kozek@yahoo.com
Keywords: hemostasis, type 2 diabetes mellitus, visceral obesity, dual energy X-ray absorptiometry
Abstract
BACKGROUND: Type 2 diabetes (T2DM) patients are characterized by a very high risk of cardiovascular diseases. Among the factors that are responsible for this phenomenon are abdominal obesity and hemostatic abnormalities. AIM OF THE STUDY: To examine the association of the markers of coagulation and fibrinolysis with the parameters of abdominal obesity and metabolic compensation in T2DM patients. METHODS: 46 T2DM patients participated in the study: 24 men (mean age 61.1 ± 7.9 years) and 22 postmenopausal women (mean age 62.6 ± 8.7 years). In each patient the content and distribution of fatty tissue was measured by a dual energy X-ray absorptiometry method (DEXA). The central abdominal fat/gynoid hip fat (CAF/GF) ratio was calculated. The following hemostatic parameters were measured: fibrinogen (Fb), factor VII (fVII), antithrombin III (ATIII), C protein (pC), tissue plasminogen activator inhibitor (PAI-1) and alpha 2 antiplasmin (alpha2 AP). In addition, the biochemical indices of metabolic compensation were measured: HbA1c, glucose levels and lipids. RESULTS: Patients of both genders were divided according to median CAF/GF ratio. The activity of PAI-1 was significantly higher in women with CAF/GF ratio ≥ 0.88 as compared to those with CAF/GF < 0.88 (2.64 ± 1.28 vs. 1.61 ± 0.27 U/ml, p < 0.05). The activity of ATIII was significantly lower in men with CAF/GF ratio ≥ 1.17, as compared to those with CAF/GF < 1.17 (105.10 ± 10.02 vs. 113.42 ± 10.72 %, p < 0.05). There was a significant correlation between the CAF/GF ratio and the activity of PAI-1 in women (r = 0.30, p < 0.05). In addition, in men the CAF/GF ratio was negatively correlated with ATIII activity (r = -0.44, p < 0.05). Multiple stepwise regression analysis demonstrated independent association between the CAF/ GF ratio and the activity of PAI-1 (p < 0.001), and between the CAF/GF ratio and the activity of alpha2 AP (p < 0.01). There was an independent association between the concentration of HbA1c and the concentration of Fb (p < 0.001) and between triglycerides and the activity of fVII (p < 0.01). CONCLUSIONS: The results of our study show that the patients with T2DM and with higher markers of abdominal obesity measured by DEXA show fibrinolysis impairment and thrombinogenesis elevation compared to those with lower abdominal obesity markers. Independent factors determining hypercoagulation also include metabolic control and lipids. Hemostatic disorders place subjects with diabetes and abdominal obesity at risk of developing vascular complications.
Introduction
Abdominal (android) obesity was first proposed to be a risk factor for the development of atherosclerosis and type 2 diabetes mellitus (T2DM) over 40 years ago [1]. Metabolic alterations accompanying the visceral distribution of fat lead to arterial hypertension, dyslipidemia, insulin resistance and subsequently to T2DM [2-6]. This phenomenon is associated not only with classical atherosclerotic risk factors but also with coagulation and fibrinolysis abnormalities [7, 8]. Hypercoagulation in abdominal obesity is thought to be caused primarily by the synthesis of factors activating coagulation and inhibiting fibrinolysis (for example factor VII activator and the fibrinolytic inhibitor PAI-1) in adipose tissue [9, 10]. Hemostatic abnormalities may also result from the synthesis in adipose tissue of cytokines that are mediators of inflammation and insulin resistance, such as interleukin 6 and TNF-alpha [7]. In addition to this direct effect, the metabolic and lipid alterations that accompany obesity and T2DM are likely to indirectly influence coagulation properties in these patients. Other factors that may be implicated in the generation of hypercoagulation state in visceral obesity and T2DM are endothelial injury and dysfunction [9]. Computerized tomography and magnetic resonance imaging are the most specific and accurate methods for measuring fat distribution and diagnosing abdominal obesity. In recent years dual energy X-ray absorptiometry (DEXA) has also been used as a standard technique for analyzing body composition and fat distribution [6, 11-18]. It was found that the assessment of adipose tissue at L1-L4 might be an indicator of glucose intolerance and lipid abnormalities [16]. Comparative studies by DEXA, computerized tomography and magnetic resonance imaging demonstrated the concordance of both techniques in the assessment of fat distribution [6, 12, 15, 17]. As shown by magnetic resonance imaging, L2-L4 is the region of the highest intra-abdominal fat and the lowest subcutaneous fat [6, 17]. The DEXA technique allows for the assessment of fat distribution and, on this basis, provides information on insulin resistance and metabolic disorders [6, 14, 15, 16].
The purpose of the present study was the assessment of the relationship of hemostatic factors with fat content and distribution as measured by the DEXA method and with parameters of metabolic compensation in patients with T2DM.
Material and Methods
Study population
46 T2DM patients participated in this study: 24 men (mean age at examination 61.1 ± 7.9 years, mean diabetes duration 11.3 ± 5.9 years) and 22 postmenopausal women (mean age 62.6 ± 8.7 years, mean diabetes duration 14.2 ± 8.7 years). All individuals with diseases that might influence the parameters of the coagulation system (liver diseases, diabetic nephropathy and renal failure, with clinically overt coronary artery disease, peripheral and cerebral vascular diseases, severe nonproliferative and proliferative diabetic retinopathy, acute and chronic infections, ketosis and ketone acidosis) were excluded from the study. Similarly, patients receiving antiplatelet agents, anticoagulants, heparin, glycosaminoglycans, glycocorticoids and hypolipemic drugs and women receiving hormone replacement therapy were not eligible. Subjects abusing alcohol were also banned from the study. Diabetes was treated as follows: 19 of the women and 17 of the men were treated with a fixed diet and insulin, 3 of the women and 7 of the men were treated with a fixed diet and sulphonylurea (gliclazide or glipizide). Each patient underwent a standard clinical examination with blood pressure measurement and an analysis of diabetic complications. Ophthalmological work-up included a full ophthalmological examination and eye fundus photography. Peripheral neuropathy was diagnosed from clinical examination with assessment of sensation and vibration. Diabetic nephropathy was assessed on the basis of screening tests for the ratio of urine albumin to creatinine levels.
Blood samples for the study of hemostatic factors were taken from the antecubital vein without clamping in the fasting state between 7 a.m. and 8 a.m. A tube containing 1 ml of 3.8% trisodium citrate and 9 ml of blood was immediately centrifuged at 4°C. Plasma was frozen at -40°C and stored for later use. Blood for HbA1c measurement was collected in a tube with EDTA as an anticoagulant. Blood was also sampled for measurement of glucose, lipids, creatinine and hepatic enzymes.
Biochemical measurements
Fibrinogen concentration was measured using a modified Clauss method on a Fibrintimer (Behringwerke). Each measurement was repeated twice and a mean was calculated. Variation coefficients were as follows: the coefficient of variation intraassay and interassay 3-6%. The activity of factor VII, ATIII, C protein, PAI-1 and alpha2 AP was measured using a ChromoTimeSystem (Behringwerke) twice and then a mean was calculated. The factor VII coefficient of variation: intra-assay and inter-assay was 4%, the AT III coefficient of variation intra-assay was 2.5-7.5%, the coefficient of variation inter-assay 5.8-8.1%, the C protein coefficient of variation intra-assay and inter-assay < 0.4%, the PAI-1 coefficient of variation intra-assay < 4%, the coefficient of variation inter-assay 3-6%, the alpha2 AP coefficient of variation intra-assay 3.2-8.5%, and the coefficient of variation inter-assay 2.9-8.8%. Concentration of HbA1c was measured by high performance liquid chromatography (Variant, BioRad). The concentrations of cholesterol and triglycerides were measured using enzymatic methods, with HDL cholesterol (HDL-ch) measured after precipitation of VLDL. The concentration of LDL cholesterol (LDL-ch) was calculated using the Friedewald formula. The level of glucose was measured using an enzymatic method.
Densitometric studies of fatty tissue
In each patient the body composition and distribution of fatty tissue was measured by dual energy X-ray absorptiometry (DEXA; Lunar DPX). The total content of adipose and soft tissue was determined. The adipose tissue in the abdominal region (central abdominal adipose tissue) was measured from the upper border of L2 to the lower border of L4. The adipose tissue of the hip region (gynoid adipose tissue) was measured as adipose tissue content between the superior iliac spines and sciatic tubers [11]. The following ratios were calculated: total fat/soft tissue (TF/ST -%), and central abdominal fat/gynoid hip fat ratio (CAF/GF).
Statistical analysis
Statistical analysis included the Students' t-test, Mann-Whitney U-test, Pearson and Spearman correlation analysis and multiple stepwise regression analysis. A p < 0.05 was considered as significant. Statistical analysis was carried out using the SAS package.
Results
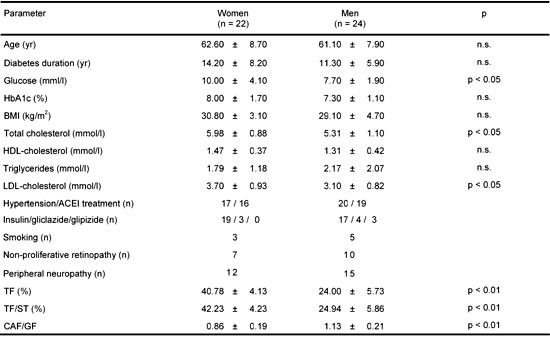
The clinical characteristics of the study group are summarized in Table 1. The mean fasting glucose concentration was significantly higher in women than in men (p < 0.05). There was no difference between genders in respect to HbA1c level. However, women had a significantly higher total cholesterol level (p < 0.05) and LDL-ch (p < 0.05) compared to men. In women, the total fat content and the TF/ST ratio was significantly higher than in men (p < 0.001 for both parameters), while the CAF/GF ratio was significantly higher in men than in women (p < 0.001 for both parameters).
Table
1.
Characteristics of the study groups |
|
 |
 |
Legend:
Data are means ± SD. BMI: body mass index. ACEI: angiotensin converting enzyme inhibitor. TF (%): total fat share. TF/ST: total fat to soft tissue ratio. CAF/GF: central abdominal fat to gynoid hip fat ratio. n: number of patients. |
|
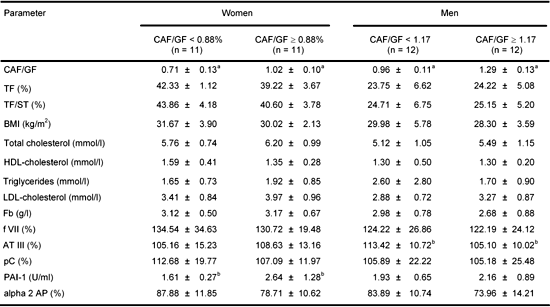
Patients were divided according to median CAF/GF ratio (Table 2). In women whose CAF/GF ratio was ≥0.88 the activity of PAI-1 was significantly higher than in the group with CAF/GF ratio < 0.88 (p < 0.05). In men whose CAF/GF ratio was ≥1.17 the activity of ATIII was significantly lower than in the group with CAF/GH ratio < 1.17 (p < 0.05). In women there was a positive correlation between the TF and alpha2AP (r = 0.43, p < 0.05), between the TF/ST ratio and alpha2 AP (r = 0.44, p < 0.05) and between the CAF/GF ratio and PAI-1 (r = 0.30, p < 0.05). Furthermore, C protein and alpha2 AP were negatively correlated with the activity of PAI-1 (r = -0.31, r = -0.36, p < 0.05 in both cases) and there was a negative correlation between diabetes duration and ATIII (r = -0.48, p < 0.05) and positive correlation between diabetes duration and PAI-1 (r = 0.48, p < 0.05). In men there was a significant negative correlation between the CAF/GF ratio and ATIII (r = -0.44, p < 0.05), a positive correlation between HbA1c and fibrinogen (r = 0.57, p < 0.05) and a negative correlation between HDL-ch and fibrinogen (r = -0.45, p < 0.05). Furthermore, there was a negative correlation between HDL-ch and alpha2 AP (r = -0.51, p < 0.05) and a positive correlation between triglycerides and factor VII (r = 0.64, p < 0.01). There was no relationship between diabetes treatment (insulin/sulphonylureas) and hemostatic factors, lipids and fat distribution parameters. Smoking had no effect, either.
Table
2.
Coagulation and fibrinolysis parameters and lipids for women and men divided according to median central abdominal fat/gynoid hip fat ratio |
|
 |
 |
Legend:
Data are means ± SD. CAF/GF: central abdominal fat to gynoid hip fat ratio. TF (%): total fat share. TF/ST: total fat to soft tissue ratio. BMI: body mass index. Fb: fibrinogen. f VII: factor VII. AT III: antithrombin III. pC: protein C. PAI-1: plasminogen activator inhibitor 1. alpha 2 AP: alpha 2 antiplasmin. a p < 0.001, b p < 0.05. |
|
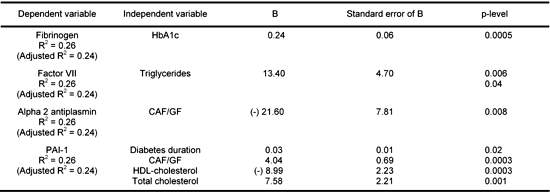
Multiple stepwise regression analysis included the following parameters: sex, age, duration of diabetes, metabolic control (HbA1c, lipids) and densitometric measurements of adipose tissue as independent variables and hemostatic parameters as dependent variables (Table 3). There was an independent relationship between fibrinogen level and HbA1c (p < 0.001) and between triglycerides and the activity of factor VII (p < 0.01). The activity of PAI-1 was independently modified by the duration of diabetes (p < 0.05), the CAF/GF ratio (p < 0.001), total cholesterol (p < 0.01) and negatively by HDL-ch (p < 0.001). There was an independent relationship between the CAF/GF ratio and the activity of alpha2 AP (p < 0.01).
Table
3.
Multiple stepwise regression analysis taking into account sex, age, duration of diabetes, metabolic control and densitometric measurements |
|
 |
 |
Discussion
The present study demonstrated the relationships between hemostatic factors and abdominal adipose tissue parameters and metabolic control in patients with T2DM.
In this study, women with an increased abdominal obesity had a higher activity of PAI-1. Furthermore, there was a correlation between the CAF/GF ratio and PAI-1 in women, and multiple step-wise regression analysis showed an independent relationship between the CAF/GF ratio and the activity of PAI-1 in women and in men. PAI-1 is the main inhibitor of fibrinolysis, and its activity is correlated with cardiovascular diseases. It should be noted that the cardiovascular risk increases more in diabetic women than in men as compared to the healthy population of both genders. One of the possible explanations is hemostatic abnormalities. Furthermore, some prospective studies in postmenopausal women showed intra-abdominal fat accumulation and increased insulin resistance [19, 20]. In our study the indices of visceral obesity were significantly lower in women than in men, whereas PAI level was correlated with the degree of obesity in the female group. This could have been influenced by the lipid abnormalities that were more profound in women. It should be noticed that in multiple regression analysis they were correlated with PAI-1. A few previously published studies showed similar results. In obese women with T2DM, increased PAI-1 was described, and this was correlated with parameters of visceral obesity measured by DEXA [22]. PAI-1 has also been found to correlate with insulin resistance parameters, BMI, and visceral fat [23, 24, 25]. However, the study by Cho did not confirm the correlation between fibrinolytic activity and obesity [26]. Martens et al. show that visceral fat is more significant than BMI or total body fat in the determination of PAI-1 levels [27]. The results by Cigolini suggest that abdominal accumulation of visceral fat is an independent predictor of plasma PAI-1 activity in healthy men [28]. In patients with visceral obesity and diabetes mellitus, fibrinolytic disorders may play a key role in atherogenesis and may be a cardiovascular risk factor.
In the present study, men with abdominal obesity had a lower activity of coagulation inhibitors ATIII which correlated with abdominal obesity indices. ATIII is an inhibitor of thrombin and factor X. In patients with obesity and diabetes, decreased ATIII activity may be a marker of thrombinogenesis and its depletion may result in inactive thrombin. It may also be related to protein inactivation in the process of non-enzymatic glycation [29]. In the present study there was no relationship between HbA1c and ATIII, only diabetes duration was significantly negatively correlated with ATIII.
Data on the relationship between C protein and obesity and diabetes is scarce and controversial [30, 31]. The study by Vegilo did not show the relationship between BMI and the activity of C protein, nor did diabetes treatment affect its activity [31]. In the present study there was no correlation between C protein activity and fat distribution parameters.
We did not find the relationship between abdominal obesity and fibrinogen concentration, whereas the concentration of Fb showed a negative correlation with HDL-ch. In another study in patients with the metabolic syndrome, there was a correlation between Fb and BMI, WHR, triglycerides, LDL-ch and HDL-ch [32, 33, 34]. In the present study, the concentration of fibrinogen correlated significantly with the level of HbA1c. There was an independent relationship between the level of HbA1c and the concentration of fibrinogen, which indicates an important effect of metabolic control on hemostatic parameters. Previously Ceriello found a relationship between the level of HbA1c and Fb in diabetes patients [35].
The present study also confirms the effect of lipids and lipoproteins on the parameters of coagulation and fibrinolysis. We found an independent relationship between triglycerides and factor VII. There was no increased activity of factor VII depending on abdominal fat distribution. Previous studies in patients with obesity measured by BMI, WHR, and DEXA show an increased activity of factor VII [22, 36-38]. A BMI-independent effect of triglycerides on factor VII was also demonstrated in individuals with hyperlipoproteinemia [37].
In our study, there was a negative relationship between abdominal obesity parameters and the activity of alpha2 AP, and a positive correlation between total fat and alpha2 AP. Alpha2 antiplasmin is the main plasma inhibitor of plasmin and a negative relationship between visceral obesity and alpha2 AP may indirectly argue in favor of increased inactivation of plasmin and inhibition of fibrinolysis. Plasmin generation is also affected by increased PAI-1 activity; the significance of this phenomenon is indirectly confirmed by the correlation between PAI-1 and alpha2 AP. Epidemiological studies show a negative correlation between the concentration of the plasmin-alpha2 AP complex and BMI, insulin, and the development of atherosclerosis; however, in patients with diabetes, the concentration of this complex was found to be increased [39, 40].
In summary, in our study, patients with T2DM and higher markers of abdominal obesity measured by DEXA showed fibrinolysis impairment and thrombinogenesis elevation compared to those with lower abdominal obesity markers. Independent factors determining hypercoagulation also include metabolic control and lipids. Hemostatic disorders place subjects with diabetes and abdominal obesity at risk of developing vascular complications.
References
- Vague J. The degree of masculine differentiation of obesities: a factor for determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956. 4:20-33. [DOD]
- Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991. 14:1132-1143. [DOD]
- Reaven GM. The role of insulin resistance and hyperinsulinemia in coronary heart disease. Metabolism 1992. 41:16-19. [DOD] [CrossRef]
- Despres JP. Abdominal obesity as an important component of insulin-resistance syndrome. Nutrition 1993. 9:452-459. [DOD]
- Rios MS. Relationship between obesity and the increased risk of major complications in non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1998. 28(suppl 2):14-18. [DOD]
- Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women. Diabetes 1996. 45:633-638. [DOD]
- Yudkin JS. Abnormalities of coagulation and fibrinolysis in insulin resistance. Evidence for a common antecedent? Diabetes Care 1999. 22(suppl 3):C25-C30. [DOD]
- Sakkinen PA, Wahl P, Cushman M, Lewis MR, Tracy RP. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol 2000. 15:897-907. [DOD] [CrossRef]
- Loskutoff DJ, Samad F. The adipocyte and hemostatic balance in obesity studies. Arterioscler Thromb Vasc Biol 1998. 18:1-6. [DOD]
- Samad F, Pandey M, Loskutoff DJ. Tissue factor gene expression in the adipose tissues of obese mice. Proc Natl Acad Sci 1998. 95:7591-7596. [DOD] [CrossRef]
- Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 1992. 55:950-954. [DOD]
- Svendsen OL, Hassager C, Bergmann I, Christiansen C. Measurement of abdominal and intra-abdominal fat in postmenopausal women by dual energy X-ray absorptiometry and anthropometry: comparison with computerized tomography. Int J Obes 1993. 17:45-51. [DOD]
- Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual energy X-ray absorptiometry. Am J Clin Nutr 1995. 61:274-278. [DOD]
- Chang Ch, Wu Ch, Lu F, Wu J, Chiu N, Yao W. Discriminating glucose tolerance status by regions of interest of dual-energy X-ray absorptiometry. Diabetes Care 1999. 22:1938-1943. [DOD]
- Kamel EG, McNeiull G, Han TS, Smith FW, Avenell A, Davidson L, Tothill P. Measurement of abdominal fat by magnetic resonance imaging, dual-energy X- ray absorptiometry and anthropometry in non-obese men and women. Int J Obes Relat Metab Disord 1999. 23:686-692. [DOD] [CrossRef]
- Paradisi G, Smith L, Burtner C, Leaming R, Garvey WT, Hook G, Johnson A, Cronin J, Steinberg HO, Baron AD. Dual energy X-ray absorptiometry assessment of fat mass distribution and its association with the insulin resistance syndrome. Diabetes Care 1999. 22:1310-1317. [DOD]
- Ross R, Shaw K, Martel Y, De Guise J, Avruch L. Adipose tissue distribution measured by magnetic resonance imaging in obese women. Am J Clin Nutr 1993. 57:470-475. [DOD]
- Park Y-W, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes 2002. 26:978-983. [DOD]
- Lemieux S, Prud'Homme D, Nadeau A, Tremblay A, Bouchard C, Despres JP. Seven-year changes in body fat and visceral adipose tissue in women. Diabetes Care 1996. 19:983-991. [DOD]
- Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord 2000. 24:226-231. [DOD] [CrossRef]
- Rissanen P, Hamalainen P, Vanninen E, Tenhunen-Eskelinen M, Uusitupa M. Relationship of metabolic variables to abdominal adiposity measured by different anthropometric measurements and dual-energy X-ray absorptiometry in obese middle-aged women. Int J Obes Relat Metab Disord 1997. 21:367-371. [DOD] [CrossRef]
- Stoney RM, Best JD, Walker KZ, O'Dea K. Regional adiposity and haemostatic profile in diabetic women and women with normal glucose tolerance. Diabetologia 1998. 41(suppl 1):A410. [DOD]
- Alessi MC, Peiretti F, Morange P, Henry M, Nalbone G, Juhan-Vague I. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 1997. 46:860-867. [DOD]
- Park YS, Park S, Park KS, Kim SY, Lee HK, Koh CS, Min HK, Kim JQ. The effect of obesity on fibrinolytic activity and plasma lipoprotein (a) levels in patients with type 2 diabetes mellitus in Korea. Diabetes Res Clin Pract 1994. 24:25-31. [DOD] [CrossRef]
- Fendri S, Roussel B, Lormeau B, Tribout B, Lalau JD. Insulin sensitivity, insulin action, and fibrinolysis activity in nondiabetic and diabetic obese subjects. Metabolism 1998. 47:1372-1375. [DOD] [CrossRef]
- Cho YW, Oh DY, Kim SJ, Hong SY, Lee HC, Huh KB. Euglobulin fibrinolytic activity in NIDDM patients. Diabetes Res Clin Pract 1991. 13:139-145. [DOD] [CrossRef]
- Mertens I, Van der Planken M, Corthouts B, Wauters M, Peiffer F, De Leeuw I, Van Gaal L. Visceral fat is a determinant of PAI-1 activity in diabetic and non-diabetic overweight and obese women. Horm Metab Res 2001. 33:602-607. [DOD] [CrossRef]
- Cigolini M, Targher G, Bergamo Andreis IA, Tonoli M, Agostino G, De Sandre G. Visceral fat Accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler Thromb Vasc Biol 1996. 16:368-374. [DOD]
- Cerriello A, Gugliano D, Quatraro A. Induced hyperglycaemia alters antithrombin III activity but not its plasma concentration in healthy normal subjects. Diabetes 1987. 36:320-325. [DOD]
- Gabazza EC, Takeya H, Deguchi H, Sumida Y, Taguchi O, Murata K, Nakatani K, Yano Y, Mohri M, Sata M, Shima T, Nishioka J, Suzuki K. Protein C activation in NIDDM patients. Diabetologia 1996. 39:1455-1461. [DOD] [CrossRef]
- Veglio M, Gruden G, Mormile A, Girotto M, Rossetto P, D'Este P, Cavallo-Perin P. Anticoagulant protein C activity in non-insulin-dependent diabetic patients with normoalbuminuria and microalbuminuria. Acta Diabetol 1995. 32:106-109. [DOD] [CrossRef]
- Imperatore G, Riccardi G, Iovine C, Rivellese A, Vaccaro O. Plasma fibrinogen: a new factor of the metabolic syndrome. Diabetes Care 1998. 21:649-654. [DOD]
- Asakawa H, Tokunaga K, Kawakami F. Relationship of abdominal fat with metabolic disorders in diabetes mellitus patients. Diabetes Res Clin Pract 2002. 55:139-149. [DOD] [CrossRef]
- Scarabin PY, Aillaud MF, Amouyel P, Evans A, Luc G, Ferrieres J, Arveiler D, Juhan-Vague I. Associations of fibrinogen, factor VII and PAI-1 with baseline findings among 10,500 male participants in a prospective study of myocardial infarction - the PRIME Study. Prospective Epidemiological Study of Myocardial Infarction. Thromb Haemost 1998. 80:749-756. [DOD]
- Ceriello A, Taboga C, Giacomello R, Falleti E, De Stasio G, Motz E, Lizzio S, Gonano F, Bartoli E. Fibrinogen plasma levels as a marker of thrombin activation in diabetes. Diabetes 1994. 43:430-432. [DOD]
- Vambergue A, Rugeri L, Gaveriaux V, Devos P, Martin A, Fermon C, Fontaine P, Jude B. Factor VII, tissue factor pathway inhibitor, and monocyte tissue factor in diabetes mellitus: influence of type of diabetes, obesity index, and age. Thromb Res 2001. 101:367-375. [DOD] [CrossRef]
- De Lorenzo F, Mukherjee M, Kadziola Z, Suleiman S, Kakkar VV. Association of overall adiposity rather than body mass index with lipids and procoagulant factors. Thromb Haemost 1998. 80:603-606. [DOD]
- Chaderevian R, Bruckert E, Dejager S, Presberg P, Turpin G. Relationship between triglycerides and factor VIIc and plasminogen activator inhibitor type-1: lack of threshold value. Thromb Res 1999. 96:175-182. [DOD] [CrossRef]
- Cortellaro M, Cofrancesco E, Boschetti C, Mussoni L, Donati M, Cordillo M, Catalano M, Gabrielli L, Lombardini B, Specchia G, Tavazzi L, Tremoli E, Pozzoli E, Turri M. Increased fibrin turnover and high PAI-1 activity as predictors of ischemic events in atherosclerotic patients: a case control study. Arterioscler Thromb 1993. 13:1412-1417. [DOD]
- Sakkineen PA, Cushman M, Psaty BM, Rodriguez B, Boineau R, Kuller LH, Tracy RP. Relationship of plasmin generation to cardiovascular disease risk factors in elderly men and women. Arterioscler Thromb Vasc Biol 1999. 19:499-504. [DOD]
This article has been cited by other articles:

|
Hyperglycaemia impairs antithrombin secretion: Possible contribution to the thrombotic risk of diabetes
Hernandez-Espinosa D, Ordonez A, Minano A, Martinez-Martinez I, Vicente V, Corral J
Thromb Res 2009. 124(4):483-489
|
|

|
Addressing the need to tailor treatment to the spectrum of type 2 diabetes: new perspectives
Rolla AR
Diabetes Technol Ther 2009. 11(5):267-274
|
|

|
Increased concentration of plasminogen activator inhibitor-1 and fibrinogen in individuals with metabolic syndrome
Palomo IG, Gutierrez CL, Alarcon ML, Jaramillo JC, Segovia FM, Leiva EM, Mujica VE, Icaza GN, Diaz NS, Moore-Carrasco R
Mol Med Rep 2009. 2(2):253-257
|
|

|
Waist circumference reduction after insulin detemir therapy in type 2 diabetes patients previously treated with NPH
Mandosi E, Fallarino M, Rossetti M, Gatti A, Morano S
Diabetes Res Clin Pract 2009. 84(2):E18-E20
|
|

|
Glycaemic control and hypoglycaemia in the PRESENT study
Almustafa M, Yeo JP, Khutsoane D
Diabetes Res Clin Pract 2008. 81(Suppl 1):S10-S15
|
|

|
Addressing Overweight and Obesity: Evolving to a Medical Consensus
Schaller FA
J Am Osteopath Assoc 2008. 108(2 Suppl 1):2-15
|
|
|