Review
| Rev Diabet Stud,
2014,
11(3-4):202-230 |
DOI 10.1900/RDS.2014.11.202 |
Adverse Effects of GLP-1 Receptor Agonists
Theodosios D. Filippatos, Thalia V. Panagiotopoulou, Moses S. Elisaf
Department of Internal Medicine, School of Medicine, University of Ioannina, 45110 Ioannina, Greece
Address correspondence to: Moses S. Elisaf, e-mail: egepi@cc.uoi.gr
Manuscript submitted January 6, 2015; resubmitted January 23, 2015; accepted February 2, 2015.
Keywords: type 2 diabetes, glucagon-like peptide-1, safety, skin, adverse effects, pancreas, kidney, cardiovascular risk, cancer
Abstract
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of injective anti-diabetic drugs that improve glycemic control and many other atherosclerosis-related parameters in patients with type 2 diabetes (T2D). However, the use of this relatively new class of drugs may be associated with certain adverse effects. Concerns have been expressed regarding the effects of these drugs on pancreatic and thyroid tissue, since animal studies and analyses of drug databases indicate an association of GLP-1 receptor agonists with pancreatitis, pancreatic cancer, and thyroid cancer. However, several meta-analyses failed to confirm a cause-effect relation between GLP-1 receptor agonists and the development of these adverse effects. One benefit of GLP-1 receptor agonists is that they do not cause hypoglycemia when combined with metformin or thiazolidinediones, but the dose of concomitant sulphonylurea or insulin may have to be decreased to reduce the risk of hypoglycemic episodes. On the other hand, several case reports have linked the use of these drugs, mainly exenatide, with the occurrence of acute kidney injury, primarily through hemodynamic derangement due to nausea, vomiting, and diarrhea. The most common symptoms associated with the use of GLP-1 receptor agonists are gastrointestinal symptoms, mainly nausea. Other common adverse effects include injection site reactions, headache, and nasopharyngitis, but these effects do not usually result in discontinuation of the drug. Current evidence shows that GLP-1 receptor agonists have no negative effects on the cardiovascular risk of patients with T2D. Thus, GLP-1 receptor agonists appear to have a favorable safety profile, but ongoing trials will further assess their cardiovascular effects. The aim of this review is to analyze critically the available data regarding adverse events of GLP-1 receptor agonists in different anatomic systems published in Pubmed and Scopus. Whenever possible, certain differences between GLP-1 receptor agonists are described. The review also provides the reader with structured data that compare the rates of the most common adverse effects for each of the various GLP-1 receptor agonists.
Abbreviations: BID – bis in die (twice a day); C-cell – parafollicular cell (in the thyroid gland); DPP-4 – dipeptidyl peptidase 4; EMA - European Medicines Agency; FAERS – FDA Adverse Event Reporting System; FDA – Food and Drug Administration; GLP-1 – glucagon-like peptide-1; Kras – Kirsten rat sarcoma viral oncogene homolog gene; KrasG12D – G12D mutation of the Kras gene; LEADER – Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; MH-OR – Mantel-Haenszel OR; OR – odds ratio; QTc interval – corrected Q wave / T wave interval; T2D – type 2 diabetes
1. Introduction
The incidence of carbohydrate metabolism derangements and many cardiovascular and renal complications is increasing [1-4]. Various classes of drugs have proved useful in the management of patients with type 2 diabetes (T2D) and its complications [1, 5-12]. Recent evidence demonstrated the beneficial effects of incretin-mimetic drugs in the treatment of T2D; these drugs include glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors [13, 14].
GLP-1 receptor agonists are characterized by increased resistance to enzymatic degradation by DPP-4 [15]. GLP-1 is secreted by the small intestine in response to nutrient ingestion. It enhances insulin secretion from pancreatic β-cells, and decreases glucagon release from pancreatic α-cells [16]. GLP-1 receptor agonists are useful, injectable drugs for the treatment of T2D as they improve glycemic control and atherosclerosis-related parameters [17-26]. Short-acting GLP-1 receptor agonists primarily slow gastric emptying, and thus exert their main effect on postprandial blood glucose levels. The long-acting compounds have insulinotropic and glucagonostatic actions, and exert their main effect on fasting glucose levels [27-29]. However, concerns have been expressed regarding their safety profile.
This review aims to discuss the available data regarding adverse effects of currently marketed GLP-1 receptor agonists.
2. Methods
We searched for eligible trials published in PubMed (last search in February 2015) by using the following search algorithm:
(Glucagon-like peptide-1 receptor agonists OR exenatide OR liraglutide OR lixisenatide OR albiglutide OR dulaglutide) AND (side effects OR adverse effects OR safety OR gastrointestinal OR pancreas OR liver OR cardiovascular OR skin OR allergy OR angioedema OR immune system OR renal OR kidney OR infection OR central nervous system OR blood OR malignancy OR cancer)
The search was limited by the following criteria:
- Published in the English language.
- Published as clinical trial, meta-analyses, case report, comparative study, observational study, evaluation study, or validation study.
The initial search identified 503 articles in Pubmed, which were scrutinized for relevance. After this initial selection, we excluded randomized clinical trials with <100 participants or with duration <12 months. Data presented in meta-analyses or large clinical trials were given more weight in the analysis than those from smaller studies. Observational and animal studies were used mainly in the sections on pancreas and cancer. Regarding the individual anatomic systems, further articles were retrieved from Pubmed and Scopus by searching relevant review articles.
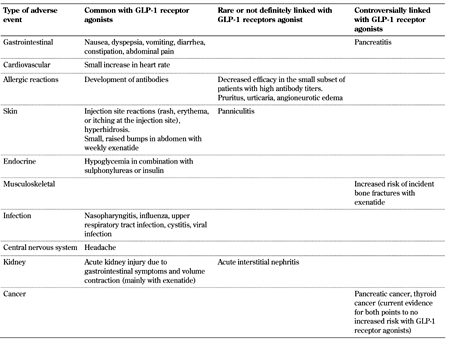
3. Adverse effects of GLP-1 receptor agonists (Table 1, Appendix)
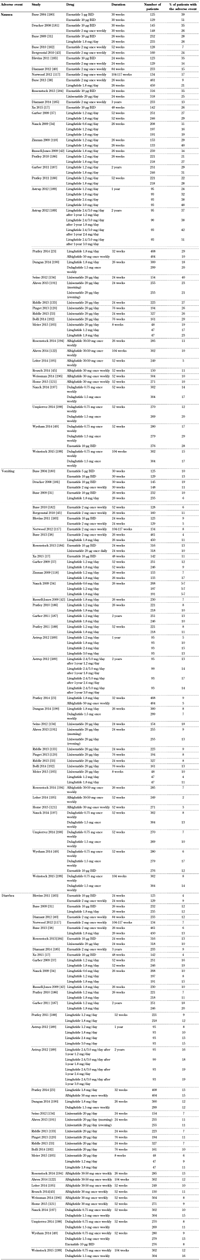
3.1 Gastrointestinal system (Table 2, Append.)
Gastrointestinal disorders were the most frequently reported adverse effects during clinical trials of GLP-1 receptor agonists [30]. Among gastrointestinal symptoms, nausea and diarrhea were very common (≥1/10), whereas vomiting, constipation, abdominal pain, and dyspepsia were relatively common (≥1/100 to <1/10) [31-45]. The frequency of these adverse effects was more pronounced at the beginning of the treatment, but gastrointestinal symptoms decreased gradually as therapy continued. Nausea is the most common adverse effect reported with GLP-1 receptor agonists; up to 50% of patients are affected. Most patients have mild to moderate episodes of nausea, which seem to be dose-dependent and diminish with ongoing treatment [46, 47]. However, it should be mentioned that exenatide treatment was discontinued in 4% of patients in clinical trials because of nausea [48]. Furthermore, dulaglutide, a newer once-weekly GLP-1 receptor agonist, was associated with increased incidence of vomiting at the dose of 1.5 mg compared with exenatide (17% vs. 12%, p < 0.05) [49].
A meta-analysis of 35 studies with exenatide and liraglutide showed that exenatide 10 µg twice-daily had a significantly higher probability of producing nausea compared with exenatide 5 µg twice-daily (odds ratio (OR): 2.28) and exenatide once-weekly (OR: 2.78) [50]. Likewise, exenatide 10 µg twice-daily had a significantly higher probability of causing nausea compared with liraglutide 1.2 mg/day (OR: 2.16) and 1.8 mg/day (OR: 3.19) [50]. Another trial in patients with T2D showed that gastric emptying is slowed to a greater degree by exenatide twice-daily than exenatide once-weekly [51]. The delay in gastric emptying has been linked to the occurrence of nausea with GLP-1 receptor agonists. More specifically, GLP-1 receptor agonists with prolonged duration of action may exert lesser effects on gastric motility, and this could be associated with less nausea. However, this is merely a hypothesis; more research is needed.
Another possible mechanism is the activation of the centers involved in appetite regulation, satiety, and nausea during the peak of the GLP-1 effect, which is evident in parallel with the injection of short-acting preparations. If nausea occurs at the peak of GLP-1 plasma concentrations, then continuous GLP-1 exposure could result in tachyphylaxis and attenuation of the pharmacological response, which in turn leads to a lower incidence of nausea and gastrointestinal symptoms [23, 52].
3.2 Pancreas
Concerns have been expressed regarding a possible association of GLP-1 receptor agonist treatment with pancreatic inflammation and pancreatitis [53-61]. In this regard, evidence from animal studies indicated a potentially harmful effect of these drugs on pancreatic tissue [62-65]. For example, administration of exenatide for 10 weeks in male rats resulted in chronic pancreatic damage in 30% of rats, characterized by pycnosis of acinar cells, increased cytoplasmic vacuoles, widened cellular gap, and inflammatory cell infiltration in pancreatic tissue [62].
Moreover, increased myeloperoxidase levels were found in pancreatic tissue of rats taking exenatide, while animals from the control and inhibitor groups did not exhibit signs of pancreatic damage [62]. Similarly, liraglutide was only modestly associated with pancreatitis risk in C57BL6 mice, whereas exendin-4 and sitagliptin were linked to signs of pancreatitis [66]. These observations point to a differential effect of incretin-mimetic drugs on the risk of pancreatitis. It should be mentioned that the findings on pancreatic tissue were mainly observed in preclinical studies aiming to find evidence of beta-cell proliferation and other diabetes-related aspects, i.e. it was not the aim of these studies to identify pancreatic damage, but they did in fact do so.
In the clinical setting, a study of 90 T2D patients taking GLP-1 receptor agonists or DPP-4 inhibitors showed that 36% of them had elevated serum amylase or lipase (or both) levels compared with 18% of T2D patients not taking these agents [67]. However, a recent analysis of the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial (n = 9340) showed that amylase and/or lipase were elevated at baseline in 22.7% of subjects [68]. More specifically, lipase levels were increased in 16.6% (n = 1540) and amylase levels in 11.8% (n = 1094) of patients, without symptoms of acute pancreatitis [68]. These findings suggest that amylase or lipase levels are a less sensitive index of pancreatic damage or pancreatitis in T2D patients.
Another study included 1,269 hospitalized cases with acute pancreatitis and 1,269 control subjects matched for age, sex, enrollment pattern, and T2D complications [69]. It was shown that the use of incretin-mimetic therapies within 30 days (adjusted OR: 2.24, 95% CI: 1.36-3.68), or their use over a period ranging from 30 days to 2 years (OR: 2.01, 95% CI: 1.37-3.18) was linked to increased risk of acute pancreatitis compared with non-users. It should be mentioned that, although the analysis was adjusted for available confounders and metformin use, the cases had increased incidence of hypertriglyceridemia, alcohol use, gallstones, tobacco abuse, obesity, biliary and pancreatic cancer, or any neoplasm [69]. These factors may lead to an increased risk of pancreatitis in patients treated with GLP-1-based therapies. A pooled analysis of phase III clinical trials and two endpoint trials showed a slightly elevated (but not significant) risk of pancreatitis with GLP-1 receptor agonists (38 events, 17,775 patient-years of exposure) compared with the alternative treatment (nine events, 5,863 patient-years of exposure; OR: 1.39 (95% CI: 0.67-2.88)) [70]. Based on these observations, the US Food and Drug Administration (FDA) investigated the risk of pancreatitis associated with the use of incretin-mimetic drugs [71].
In contrast to the above results, several recent studies and meta-analyses failed to show increased risk of pancreatitis with the use of GLP-1 receptor agonists [72-82]. A meta-analysis of 55 randomized controlled trials (n = 33,350) showed no increased risk of pancreatitis with GLP-1 agonists compared with controls (OR: 1.05, 95% CI: 0.37-2.94) [83]. Moreover, the analysis of three retrospective cohort studies and two case-control studies (total n = 320,289) did not show an increased risk of pancreatitis with the administration of exenatide or sitagliptin [83]. Likewise, another recent meta-analysis of nine observational studies (n = 1,324,515 patients and 5,195 cases of acute pancreatitis) found no significant association between incretin-based treatment and acute pancreatitis (OR: 1.03, 95% CI: 0.87-1.20) [84]. Furthermore, FDA and European Medicines Agency (EMA) recently reevaluated more than 250 toxicology studies conducted in nearly 18,000 healthy animals and more than 200 trials involving approximately 28,000 patients taking incretin-based drugs. In a joint assessment, both agencies concluded that the concerns expressed by many authors and the media regarding a possible causal association of incretin-mimetic drugs with acute pancreatitis are inconsistent with the current data [85].
Generally, no direct cause and effect relationship has been shown between GLP-1 receptor agonists and pancreatitis [86]. Moreover, T2D and hypertriglyceridemia are both independent risk factors for pancreatitis [87, 88]. The presence of confounding factors should be taken into account when investigating the possible association between GLP-1 receptor agonists and pancreatitis. However, until this matter finally resolves, it may be prudent that GLP-1 receptor agonists should not be given to patients with several risk factors for pancreatitis, such as severe hypertriglyceridemia or alcohol intake.
3.3 Cardiovascular system
The available data do not indicate any increase in cardiovascular events with GLP-1 receptor agonists [89-91]. A meta-analysis of 36 trials with a duration ≥12 weeks showed that the Mantel-Haenszel OR (MH-OR) for major cardiovascular events versus placebo or other comparators was 0.74 for all GLP-1 receptor agonists (95% CI: 0.50-1.08, p = 0.12), 0.85 for exenatide (95% CI: 0.50-1.45, p = 0.55) and 0.69 for liraglutide (95% CI: 0.40-1.22, p = 0.20) [92]. Specifically designed long-term trials are currently assessing the cardiovascular effects of GLP-1 receptor agonists.
GLP-1 receptor agonist treatment has however been associated with a small increase in heart rate [93] (Table 3, in the Appendix). A meta-analysis of 22 trials showed that GLP-1 agonists overall resulted in a significant increase in heart rate with a weighted mean difference of 1.86 beats per minute (bpm, 95% CI: 0.85-2.87) compared with placebo and 1.90 bpm (95% CI: 1.30-2.50) compared with active control (all p < 0.05) [94]. Exenatide twice-daily increased the heart rate by 0.82 bpm (95% CI: -0.15 to 1.79) compared with active control and by 0.88 bpm (95% CI: -0.47 to 2.22) compared with placebo (both p > 0.05). In a small number of studies with exenatide once-weekly, the drug increased heart rate by 2.14 bpm (95% CI: 1.11-3.17) compared with active control (p < 0.05). Liraglutide increased heart rate by 2.71 bpm (95% CI: 1.45-3.97) compared with placebo and 2.49 (95% CI 1.77-3.21) compared with active control (all p < 0.05) [94].
Dulaglutide, a newer once-weekly GLP-1 receptor agonist, significantly increased heart rate (2.8 bpm, 95% CI: 1.5-4.2) compared with placebo at the dose of 1.5 mg [95]. Interestingly, a 28-day study showed that lixisenatide significantly reduced the heart rate by 3.6 bpm, whereas liraglutide increased heart rate by 5.3 bpm (mean difference 8.9 bpm, p < 0.05) [96]. Moreover, in another face to face 26-week study, dulaglutide 1.5 or 0.75 mg significantly increased heart rate compared with exenatide 10 µg BID (2.8 vs. 1.2 beats per minute, p < 0.05) [49]. Generally, the increase in heart rate with GLP-1 receptor agonists is small but clinically relevant as heart rate is a marker of cardiovascular disease [97].
Exenatide, liraglutide, and albiglutide do not cause any clinically relevant increase in the QTc interval [98-101]. Exenatide does not prolong QTc even at supratherapeutic concentrations [102].
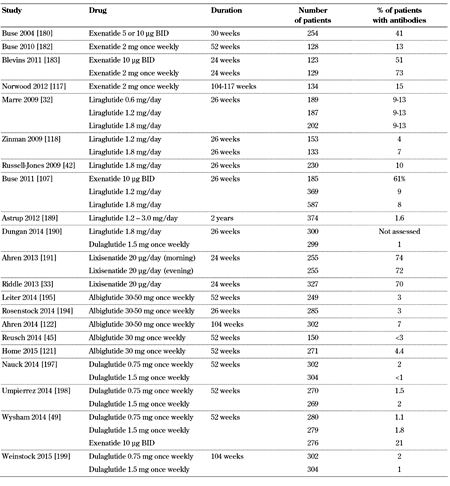
3.4 Allergy and angioedema
GLP-1 receptor agonists are synthetic peptides and, like other subcutaneously injected peptides, may lead to antibody formation (Table 4, in the Appendix). The incidence of antibody formation was 44% with exenatide, 8.6% with liraglutide, 69.8% with lixisenatide, 4% with albiglutide, and 1.6% with dulaglutide [48, 103-106]. These data show that the various GLP-1 receptor agonists have different immunogenicity [107]. A multicenter, open-label, 24-week study assessed the incidence of immune-related and hypersensitivity reactions after exenatide re-exposure in 58 patients with T2D [108]. Treatment-emergent adverse events were observed in 40% and 47% of patients with positive and negative treatment-emergent antibodies, respectively. Immune-related adverse events, which were not observed previously, appeared in 4 patients with positive and 2 with negative treatment-emergent antibodies. However, re-exposure to exenatide was not associated with increased hypersensitivity reactions [108].
In an analysis of 12 controlled (n = 2,225; duration 12-52 weeks) and five uncontrolled (n = 1,538; duration up to 3 years) exenatide twice-daily trials, and four controlled (n = 653; duration 24-30 weeks) exenatide once-weekly trials with one uncontrolled period (n = 128), the antibody titers peaked early and subsequently declined [109]. Specifically, after 30 weeks, 36.7% of patients receiving exenatide twice-daily were antibody-positive. Most of the antibody-positive patients (31.7%) had low titers, but 5% had high antibody titers. After three years, only 16.9% were antibody-positive, with 1.4% having high titers. Similarly, 56.8% of patients receiving exenatide once-weekly were antibody-positive (11.8% with high titer) at 24-30 weeks, and this percentage was reduced to 45.4% (9.2% with high titer) at 52 weeks. Adverse event rates were similar between antibody-positive and antibody-negative patients, with the exception of injection site reactions. Importantly, as shown in a subset of patients, no significant cross-reaction was observed between anti-exenatide antibodies and human GLP-1 or glucagon. Moreover, efficacy was similar in the entire cohort of antibody-positive and antibody-negative patients (HbA1c change: -0.9% and -1.0%, respectively, with exenatide twice-daily; -1.3% and -1.6% with exenatide once-weekly). However, the reduction in HbA1c was non-significantly diminished with exenatide twice-daily in the small subset of patients (5%) with higher titers. Additionally, a significant decrease in efficacy was observed in the subset of patients who had high antibody titers and who received exenatide once-weekly (12%) [109]. These results represent a clinically relevant discovery, pointing to diminishing efficacy of exenatide in patients who develop high titers of emergent-treatment antibodies.
Severe anaphylactic reactions with GLP-1 receptor agonists have not been reported in Pubmed. However, post-marketing reports show that anaphylactic reactions occur rarely with liraglutide (≥1/10,000 to <1/1,000), very rarely with exenatide (<1/10,000), and uncommonly with lixisenatide (≥1/1,000 to <1/100) [48, 104, 105, 110]. Moreover, rare post-marketing reports of pruritus, urticaria, and angioneurotic edema have been described with exenatide, liraglutide, and lixisenatide [48, 104, 105]. Dulaglutide was linked to systemic hypersensitivity events in 0.5% of patients in phase II and III trials [103].
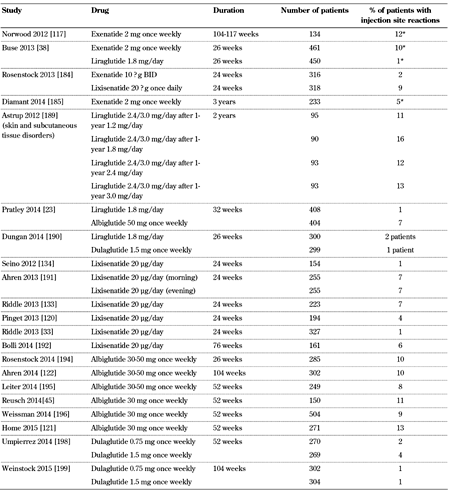
3.5 Skin side effects
Injection site reactions, such as rash, erythema, or itching at the injection site, are common with GLP-1 receptor agonists (Table 5, in the Appendix). In phase II and III trials, 5.1% of the patients receiving exenatide twice-daily, 16% of those receiving exenatide once-weekly, 3.9% of those receiving lixisenatide, and 15% of those receiving albiglutide experienced injection site reactions [48, 105, 106, 110]. Injection site reactions are reported more frequently with long-acting than short-acting GLP-1 receptor agonists [111]. The predominant injection site reaction is pruritus. These reactions are most often transient, and generally do not cause discontinuation of the treatment. Interestingly, patients who receive GLP-1 receptor analogs, and who develop antibodies against the drug, tend to have more injection site reactions, despite the fact that they experience similar rates and types of adverse events to those experienced by patients who do not develop antibodies [48, 105, 106, 109, 110].
Many patients receiving exenatide once weekly experience small, raised bumps in their abdomen, which usually have a diameter of less than 0.75 cm. This reaction is attributed to the known properties of poly (D,L-lactide co-glycolide) polymer microsphere formulation of the drug. Generally, despite this reaction, which typically resolves within 4-8 weeks, patients remain asymptomatic, and do not discontinue the drug [110].
In some case reports, exenatide administration has been associated with the development of panniculitis, although a causal association has not been proven [112-114]. Hyperhidrosis was reported as a common adverse event (≥1/100 to < 1/10), while alopecia and macular/papular rash were found to be rare (≥1/10,000 to < 1/1,000), in phase III trials of exenatide [48].
3.6 Endocrine effects
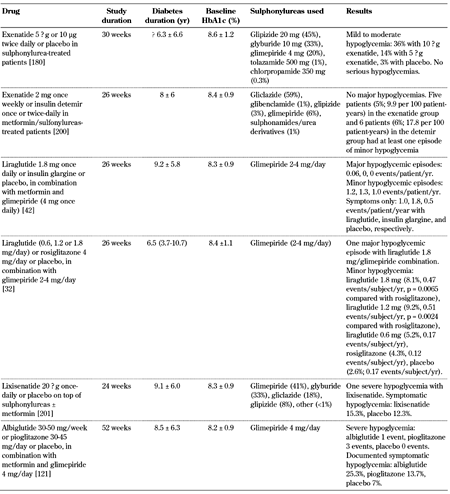
The combination of GLP-1 receptor agonists and metformin has not been associated with an increase in the rate or severity of clinically relevant hypoglycemic events [32, 34, 45, 46, 115-122]. However, in clinical trials examining GLP-1 receptor agonists in combination with sulphonylurea (with or without metformin) or insulin, the incidence of hypoglycemia, although low overall, was increased compared with placebo (Table 6, in the Appendix) [32, 33, 42, 123-135]. Interestingly, the concomitant administration of GLP-1 receptor agonists with a sulphonylurea in the in situ perfused rat pancreas led to uncoupling of the insulinotropic effect of GLP-1 from its glucose dependence [136]. Hence, the increased incidence of hypoglycemia caused by the combination of GLP-1 receptor agonists and sulphonylureas may be linked to the uncoupling of GLP-1 from its glucose dependence. Therefore, it is recommendable to decrease the sulphonylurea or insulin dose in patients on sulphonylurea or insulin therapy who start a GLP-1 receptor agonist to reduce the risk of hypoglycemic episodes [137].
3.7 Musculoskeletal disorders
A meta-analysis of 16 randomized controlled trials (n = 11,206) assessed the risk of bone fractures associated with liraglutide or exenatide, in comparison to placebo or other active drugs [138]. The administration of liraglutide was associated with a significantly decreased risk of incident bone fractures (MH-OR: 0.38, 95% CI: 0.17-0.87), while exenatide administration was linked with an increased risk (MH-OR: 2.09, 95% CI: 1.03-4.21). These results point to heterogeneity between liraglutide and exenatide. However, these observations need to be confirmed by future trials [138].
Despite the negative effects of exenatide on bone fracture risk found in the above meta-analysis, a study of 69 metformin-treated subjects with T2D randomized to exenatide twice-daily (n = 36) or insulin glargine once-daily (n = 33) showed that bone mineral density was similar in both groups after the 44-week therapy (between-group difference p = 0.782). Moreover, fasting serum alkaline phosphatase, calcium, and phosphate levels did not significantly change during treatment [139].
3.8 Infection
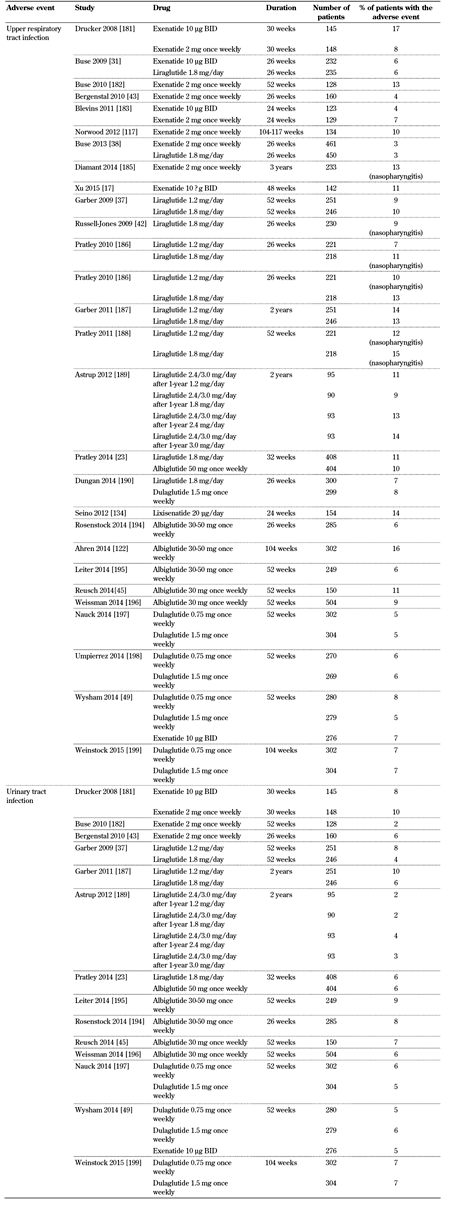
GLP-1 receptor agonist trials report upper respiratory and urinary tract infections (Table 7, in the Appendix). Nasopharyngitis, influenza, cystitis, and viral infection are also commonly reported with these drugs [48, 104-106, 110]. However, no cause-effect association between the use of GLP-1 receptor agonists and more serious infections has been observed.
3.9 Central nervous system (CNS)
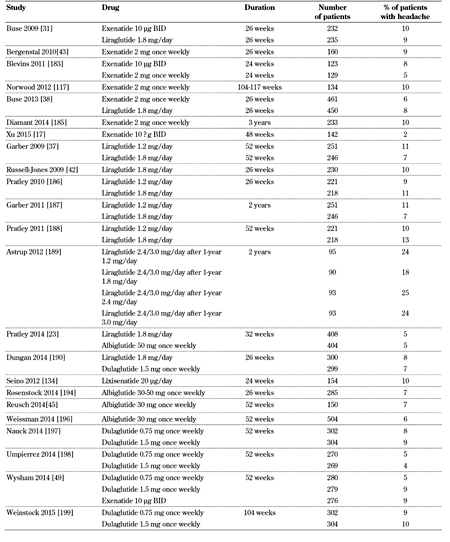
Headache is reported in GLP-1 receptor agonist trials (Table 8, in the Appendix). However, this adverse effect does not usually lead to discontinuations.
3.10 Renal effects
Exenatide has been associated with the development of acute kidney injury in about 100 case reports [140-146]. In most reports, acute kidney injury was attributed to pre-renal acute failure due to exenatide-induced nausea and vomiting, decreased fluid intake, and significant loss of fluids. In this context, a kidney biopsy was performed and showed ischemic glomeruli with moderate to severe interstitial fibrosis and early diabetic nephropathy [145]. Other factors that may be associated with the impairment of renal function are GLP-1-induced natriuresis and reduction in renal perfusion [147, 148].
It should be mentioned that most of the subjects who experienced deterioration of kidney function with exenatide had at least one risk factor for developing kidney disease, such as cardiac failure, hypertension, or use of nephrotoxic drugs [140]. Importantly, most subjects with exenatide-induced volume contraction were receiving drugs that inhibit the renin-angiotensin system and aldosterone formation. Activation of the renin-angiotensin system is an important homeostatic mechanism in states associated with volume depletion, and the use of inhibitors of these systems contributes to occurrence of acute kidney injury in subjects with volume depletion [149].
Acute interstitial nephritis has also been demonstrated to be a mechanism of exenatide-induced acute kidney injury. In a case report on a person with exenatide-associated acute kidney injury, kidney biopsy showed active, moderately severe, diffuse tubulointerstitial nephritis with infiltration of eosinophils [150]. These observations suggested a drug-induced reaction. The person’s condition was improved with the administration of prednisolone [150]. Another case report showed an improvement in kidney function with prednisolone in a person with exenatide-induced acute kidney injury [151].
In contrast to the case reports on altered renal function seen with exenatide, a pooled analysis of 5,594 participants from 19 randomized, controlled clinical trials of exenatide twice-daily (5 μg and 10 μg) showed that renal impairment-related adverse events, including acute renal failure, were rare (1.6 per 100 person-years for both groups). Also, no significant difference was seen between the exenatide and pooled comparator (placebo and insulin) groups (95% CI: -0.98 to 0.96) [152]. Further analyses have shown that there was no difference in the adjusted risk for acute kidney injury among T2D patients taking exenatide compared with patients receiving other drugs (hazard ratio (HR): 0.77, 95% CI: 0.42-1.41, p = 0.40) [153].
Liraglutide has been associated with acute kidney injury in a few case reports [140, 154]. The pathological mechanism seems to be based on acute tubular necrosis due to dehydration and volume contraction resulting from severe and progressively worsening gastrointestinal symptoms [155]. Liraglutide has been linked to acute interstitial nephritis in one case report [156]. Despite these few reports, liraglutide seems to be a safe drug in terms of kidney function.
Clinicians should consider the possible risk of acute renal failure associated with the administration of GLP-1 receptor agonists. The main mechanism of acute kidney injury in people receiving GLP-1 receptor agonists is volume contraction due to gastrointestinal symptoms [140]. Therefore, these drugs should not be given to people with uncontrolled T2D, polyuria, and polydipsia or people who develop severe symptoms that predispose to volume depletion (for example vomiting). Patients should also be advised to discontinue therapy in case of severe vomiting or diarrhea.
Clinicians should also be cautious in the case of T2D patients receiving drugs that inhibit the renin-angiotensin system. These patients are more prone to develop acute kidney injury due to dehydration and volume contraction. GLP-1 receptor agonists should be stopped in patients with acute kidney injury, and rehydration should be initiated. In severe cases, dialysis may be needed. Finally, in cases where volume contraction is not the obvious mechanism of acute kidney injury, the possibility of acute interstitial nephritis should be considered. In such a case, a kidney biopsy should be performed to establish the diagnosis and start steroid treatment.
3.11 Cancer
Concerns have been expressed about the effects of incretin-mimetic drugs on pancreatic tissue [157, 158]. It has been shown that the administration of the GLP-1 receptor agonist exendin-4 results in the expansion of pancreatic duct glands in rats, and acceleration of the formation of dysplastic lesions and chronic pancreatitis in the KrasG12D mouse model [63].
The results of animal studies are difficult to extrapolate to humans. A study by Butler et al. examined pancreatic tissue from organ donors with T2D taking sitagliptin (n = 7), exenatide (n = 1), or other medication (n = 12), and from a group of subjects without diabetes (n = 14). In 40% of patients, treatment with incretin-mimetic drugs was associated with increased pancreatic mass accompanied by increased exocrine cell proliferation (p < 0.0001) and increased pancreatic intraepithelial neoplasia (dysplasia, p < 0.01) [159]. Moreover, patients receiving incretin-mimetic drugs had α-cell hyperplasia and glucagon-expressing microadenomas (3 out of 8) and one had a neuroendocrine tumor [159].
Analyses based on the FDA Adverse Event Reporting System (FAERS) showed that incretin-mimetic drugs are linked with increased incidence of pancreatitis and pancreatic cancer compared with other anti-diabetic drugs [160-162]. A FAERS analysis conducted between 2004 and 2009 showed a significant increase in cases of pancreatitis with exenatide (OR: 10.68, 95% CI: 7.75-15.1, p < 0.0001) and sitagliptin (OR: 6.74, 95% CI: 4.61-10.0, p < 0.0001), and in cases of pancreatic cancer (exenatide OR: 2.9, p < 0.0001; sitagliptin OR: 2.7, p = 0.008) and thyroid cancer (exenatide OR: 4.73, p = 0.004) [161]. Based on this evidence, FDA investigates reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin-mimetic drugs [71].
However, it should be mentioned that the study by Butler et al. has been accused of methodological deficiencies including the following issues:
- The age distribution of the three groups was mismatched.
- The increase in pancreatic mass was due to an outlier in the GLP-1 receptor agonists group.
- The increase in pancreatic mass may also be due to the possible inclusion of type 1 diabetic patients (who have decreased pancreatic mass) in the type 2 diabetic control group [163].
- The distinction between alpha- and beta-cells was not clear due to variability in staining intensity [163].
Other long-term animal studies did not find a harmful effect of incretin-mimetic drugs on pancreatic tissue [164, 165]. Furthermore, analyses from adverse reporting systems are useful, but are difficult to control for confounding factors that increase the risk of pancreatitis and cancer, such as obesity, alcohol consumption, smoking, and the use of other drugs. Recent reports and meta-analyses did not show an increased risk of pancreatic cancer with incretin-mimetic drugs [77, 166, 167]. A meta-analysis of 25 studies showed that the use of exenatide (OR: 0.86, 95% CI: 0.29-2.60) and liraglutide (OR: 1.35, 95% CI: 0.70-2.59) did not significantly increase the risk of pancreatic cancer, independently of the baseline comparator [78].
Hence, at this point no significant evidence exists linking GLP-1 receptor agonists with pancreatic cancer. In a recent joint statement, FDA and EMA agree that concerns regarding a causal association between incretin-mimetic drugs and pancreatitis or pancreatic cancer are inconsistent with the available evidence [85]. However, a final all-clear regarding the risk of pancreatitis or pancreatic cancer caused by incretin-mimetic drugs cannot be given [85]. On the other hand, there is some evidence of an antitumor effect of GLP-1 receptor activation in pancreatic cancer cell lines [168].
Concerns have also been expressed regarding a possible link between GLP-1 receptor agonists and medullary thyroid cancer [161]. These concerns are mainly based on rodent studies. Liraglutide and exenatide have been associated with the development of thyroid C-cell tumors in rodents after lifetime exposure at supratherapeutic doses [169]. Furthermore, a 13-week continuous exposure to GLP-1 receptor agonists induced marked increases in plasma calcitonin and in the incidence of C-cell hyperplasia in wild-type mice, but not in GLP-1 receptor knockout mice [170]. In contrast to the results in rodents, in vivo animal studies with cynomologus monkeys did not show any liraglutide-induced calcitonin release or any effect on relative C-cell fraction in the thyroid gland after 87 weeks at doses up to 60-fold higher than the highest dose recommended in humans [169]. Another study in monkeys also showed that dulaglutide at doses amounting to the 500-fold maximum human plasma exposure for 52 weeks were not associated with increases in serum calcitonin or alterations in thyroid weight, histology, C-cell proliferation, or absolute/relative C-cell volume [171].
The harmful effect of GLP-1 receptor agonists in rodents but not in primates indicates that the proliferative C-cell effects may be rodent-specific [172]. Moreover, an analysis of liraglutide trials (duration 20-104 weeks) did not show any significant change between treatment groups regarding calcitonin levels or the proportion of participants with an increase in calcitonin levels above 20 pg/ml [173]. It should be mentioned that in the Liraglutide Effect and Action in Diabetes (LEAD)-6 trial (duration 26 weeks) no difference was observed in estimated geometric mean calcitonin levels between liraglutide 1.8 mg once daily and exenatide 10 μg twice daily [31]. Finally, a meta-analysis of 25 studies showed that liraglutide was not significantly associated with an increased risk of thyroid cancer (OR: 1.54, 95% CI: 0.40-6.02), and no thyroid malignancies were reported with exenatide [78].
3.12 Overdose
Some cases of GLP-1 receptor agonist overdose have been reported [174-178]. For example, a 49-year-old woman with T2D accidentally injected the whole liraglutide pen and received a total dose of 18 mg [179]. She developed severe nausea and vomiting, but no abdominal pain, deterioration of liver function tests, increase in amylase levels, or hypoglycemia. This patient was treated with intravenous fluids and intravenous metoclopramide [179].
Generally, severe nausea, vomiting, abdominal pain, and diarrhea were described in cases of GLP-1 receptor agonist overdose. Hypoglycemia did not develop, even with doses of 72 mg or in cases where an overdose of liraglutide was continued for seven months [174, 175]. Moreover, no incident of pancreatitis or other serious complication has been reported. Therapy in GLP-1 receptor agonist overdose is mainly supportive.
4. Conclusions
GLP-1 receptor agonists are useful drugs for the treatment of patients with T2D. These drugs improve glycemic control and many other atherosclerosis-related parameters. However, concerns have been expressed regarding the effects of these drugs on pancreatic and thyroid tissue, but current evidence and meta-analyses do not show a cause-effect association between GLP-1 receptor agonists and the development of pancreatitis, pancreatic cancer, or thyroid cancer.
GLP-1 receptor agonists do not generally cause hypoglycemia, but it is recommendable to decrease the dose of concomitant sulphonylurea or insulin to reduce the risk of hypoglycemic episodes. In many case reports, the use of these drugs, mainly exenatide, has been associated with acute kidney injury, in which hemodynamic factors are predominantly implicated. Other common adverse effects of these drugs include injection site reactions, headache, and nasopharyngitis, but these effects do not usually lead to discontinuation of the drug.
Finally, GLP-1 receptor agonists do not seem to affect negatively the cardiovascular risk in patients with T2D, but an ultimate all-clear regarding the risk of cardiovascular events, pancreatitis, or pancreatic cancer, which may be caused by incretin-mimetic drugs, cannot be given as there is scattered evidence for these side effects. Ongoing and future trials need to assess and clarify further the cardiovascular and overall safety profile of GLP-1 receptor agonists.
5. Article highlights
- GLP-1 receptor agonists cause gastrointestinal symptoms, but these effects do not usually cause discontinuation of therapy.
- Nausea appears to be less common with long-acting than short-acting compounds.
- GLP-1 receptor agonists are associated with pre-renal acute kidney injury in cases of severe gastrointestinal symptoms and dehydration.
- Evidence does not point to an increased risk of pancreatitis with GLP-1 receptor agonists.
- Meta-analyses do not show an increased risk of pancreatic or thyroid cancer with the use of these drugs.
Disclosures
This work was conducted independently; it was not supported financially. Professor Moses Elisaf has received speaker honoraria, consulting fees, and research funding from AstraZeneca, Schering Plough, Merck, Pfizer, Solvay, Abbott, Boehringer Ingelheim, and Fournier, and has participated in clinical trials with AstraZeneca, Merck, Sanofi-Synthelabo, Solvay, Glaxo, Novartis, Pfizer, and Fournier. The authors have given talks and attended conferences sponsored by various pharmaceutical companies, including Bristol-Myers Squibb, Pfizer, Lilly, Abbott, Amgen, Astrazeneca, Novartis, Vianex, Teva, and MSD.
Appendix
Table
1.
Adverse effects of GLP-1 receptor agonists |
|
 |
 |
Table
2.
Incidence of nausea, vomiting, and diarrhea associated with GLP-1 receptor agonists |
|
 |
 |
Table
3.
Heart rate increase with GLP-1 receptor agonists |
|
 |
 |
Table
4.
Treatment-emergent antibodies to GLP-1 receptor agonists |
|
 |
 |
Table
5.
Injections site reactions with GLP-1 receptor agonists |
|
 |
 |
Legend:
* Injection site nodule. |
|
Table
6.
Incidence of hypoglycemia in selected placebo-controlled studies with GLP-1 receptor agonists combined with sulphonylureas |
|
 |
 |
Table
7.
Incidence of upper respiratory and urinary tract infection with GLP-1 receptor agonists |
|
 |
 |
Table
8.
Incidence of headache with GLP-1 receptor agonists |
|
 |
 |
References
- American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care 2014. 37(Suppl 1):S14-S80. [DOD] [CrossRef]
- Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Pitaraki E, Metaxa V, Stefanadis C, Attica Study Group. 10-year incidence of diabetes and associated risk factors in Greece: the ATTICA study (2002-2012). Rev Diabet Stud 2014. 11(2):181-189. [DOD] [CrossRef]
- Filippatos TD, Rizos EC, Gazi IF, Lagos K, Agouridis D, Mikhailidis DP, Elisaf MS. Differences in metabolic parameters and cardiovascular risk between American Diabetes Association and World Health Organization definition of impaired fasting glucose in European Caucasian subjects: a cross-sectional study. Arch Med Sci 2013. 9(5):788-795. [DOD] [CrossRef]
- Filippatos TD, Rizos EC, Tsimihodimos V, Gazi IF, Tselepis AD, Elisaf MS. Small high-density lipoprotein (HDL) subclasses are increased with decreased activity of HDL-associated phospholipase A(2) in subjects with prediabetes. Lipids 2013. 48(6):547-555. [DOD] [CrossRef]
- Agouridis AP, Rizos CV, Elisaf MS, Filippatos TD. Does combination therapy with statins and fibrates prevent cardiovascular disease in diabetic patients with atherogenic mixed dyslipidemia? Rev Diabet Stud 2013. 10(2-3):171-190. [DOD]
- Filippatos TD, Elisaf MS. Combination drug treatment in obese diabetic patients. World J Diabetes 2010. 1(1):8-11. [DOD] [CrossRef]
- Opie LH. Sodium glucose co-transporter 2 (SGLT2) inhibitors: new among antidiabetic drugs. Cardiovasc Drugs Ther 2014. 28(4):331-334. [DOD] [CrossRef]
- Agouridis AP, Filippatos TD, Derdemezis CS, Mikhailidis DP, Elisaf MS. Combination of fenofibrate with non-statin drug regimens. Curr Pharm Des 2010. 16(30):3401-3416. [DOD] [CrossRef]
- Filippatos T, Milionis HJ. Treatment of hyperlipidaemia with fenofibrate and related fibrates. Expert Opin Investig Drugs 2008. 17(10):1599-1614. [DOD] [CrossRef]
- Filippatos TD, Elisaf MS. Fenofibrate plus simvastatin (fixed-dose combination) for the treatment of dyslipidaemia. Expert Opin Pharmacother 2011. 12(12):1945-1958. [DOD] [CrossRef]
- Filippatos TD, Kiortsis DN, Liberopoulos EN, Georgoula M, Mikhailidis DP, Elisaf MS. Effect of orlistat, micronised fenofibrate and their combination on metabolic parameters in overweight and obese patients with the metabolic syndrome: the FenOrli study. Curr Med Res Opin 2005. 21(12):1997-2006. [DOD] [CrossRef]
- Filippatos TD, Liberopoulos EN, Kostapanos M, Gazi IF, Papavasiliou EC, Kiortsis DN, Tselepis AD, Elisaf MS. The effects of orlistat and fenofibrate, alone or in combination, on high-density lipoprotein subfractions and pre-beta1-HDL levels in obese patients with metabolic syndrome. Diabetes Obes Metab 2008. 10(6):476-483. [DOD] [CrossRef]
- Simsek S, de Galan BE. Cardiovascular protective properties of incretin-based therapies in type 2 diabetes. Curr Opin Lipidol 2012. 23(6):540-547. [DOD] [CrossRef]
- Filippatos TD, Athyros VG, Elisaf MS. The pharmacokinetic considerations and adverse effects of DPP-4 inhibitors. Expert Opin Drug Metab Toxicol 2014. 10(6):787-812. [DOD] [CrossRef]
- Neumiller JJ. Clinical pharmacology of incretin therapies for type 2 diabetes mellitus: implications for treatment. Clin Ther 2011. 33(5):528-576. [DOD] [CrossRef]
- Eckerle Mize DL, Salehi M. The place of GLP-1-based therapy in diabetes management: differences between DPP-4 inhibitors and GLP-1 receptor agonists. Curr Diab Rep 2013. 13(3):307-318. [DOD] [CrossRef]
- Xu W, Bi Y, Sun Z, Li J, Guo L, Yang T, Wu G, Shi L, Feng Z, Qiu L, et al. Comparison of the effects on glycaemic control and beta-cell function in newly diagnosed type 2 diabetes patients of treatment with exenatide, insulin or pioglitazone: a multicentre randomized parallel-group trial (the CONFIDENCE study). J Intern Med 2015. 277(1):137-150. [DOD] [CrossRef]
- Tzanetakos C, Melidonis A, Verras C, Kourlaba G, Maniadakis N. Cost-effectiveness analysis of liraglutide versus sitagliptin or exenatide in patients with inadequately controlled type 2 diabetes on oral antidiabetic drugs in Greece. BMC Health Serv Res 2014. 14:419. [DOD] [CrossRef]
- Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev 2014. 30(6):521-529. [DOD] [CrossRef]
- McCormack PL. Exenatide twice daily: a review of its use in the management of patients with type 2 diabetes mellitus. Drugs 2014. 74(3):325-351. [DOD] [CrossRef]
- Scott LJ. Liraglutide: a review of its use in adult patients with type 2 diabetes mellitus. Drugs 2014. 74(18):2161-2174. [DOD] [CrossRef]
- Rizzo M, Chandalia M, Patti AM, Di Bartolo V, Rizvi AA, Montalto G, Abate N. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc Diabetol 2014. 13:49. [DOD] [CrossRef]
- Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, Ye J, Scott R, Johnson S, Stewart M, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014. 2(4):289-297. [DOD] [CrossRef]
- Okada K, Kotani K, Yagyu H, Ando A, Osuga J, Ishibashi S. Effects of treatment with liraglutide on oxidative stress and cardiac natriuretic peptide levels in patients with type 2 diabetes mellitus. Endocrine 2014. 47(3):962-964. [DOD] [CrossRef]
- Muscogiuri G, Gastaldelli A. Albiglutide for the treatment of type 2 diabetes. Drugs Today (Barc) 2014. 50(10):665-678. [DOD] [CrossRef]
- Scott LJ. Lixisenatide: a review of its use in patients with type 2 diabetes mellitus. BioDrugs 2013. 27(5):509-523. [DOD] [CrossRef]
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012. 8(12):728-742. [DOD] [CrossRef]
- Marathe CS, Rayner CK, Jones KL, Horowitz M. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Exp Diabetes Res 2011. 2011:279530. [DOD] [CrossRef]
- Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003. 88(6):2719-2725. [DOD] [CrossRef]
- Sun F, Chai S, Yu K, Quan X, Yang Z, Wu S, Zhang Y, Ji L, Wang J, Shi L. Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Technol Ther 2015. 17(1):35-42. [DOD] [CrossRef]
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L, LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009. 374(9683):39-47. [DOD] [CrossRef]
- Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S, LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 2009. 26(3):268-278. [DOD] [CrossRef]
- Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, Ping L, Ye J, Rosenstock J. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013. 36(9):2489-2496. [DOD] [CrossRef]
- Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, During M, Matthews DR, LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009. 32(1):84-90. [DOD] [CrossRef]
- Nauck M, Frid A, Hermansen K, Thomsen AB, During M, Shah N, Tankova T, Mitha I, Matthews DR. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab 2013. 15(3):204-212. [DOD] [CrossRef]
- Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007. 50(2):259-267. [DOD] [CrossRef]
- Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B, LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009. 373(9662):473-481. [DOD] [CrossRef]
- Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 2013. 381(9861):117-124. [DOD] [CrossRef]
- Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab 2011. 13(4):348-356. [DOD] [CrossRef]
- Diamant M, Van Gaal L, Stranks S, Guerci B, MacConell L, Haber H, Scism-Bacon J, Trautmann M. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care 2012. 35(4):683-689. [DOD] [CrossRef]
- Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, Gonzalez JG, Chan M, Wolka AM, Boardman MK. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care 2012. 35(2):252-258. [DOD] [CrossRef]
- Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R, Liraglutide E, LEAD-5 Met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009. 52(10):2046-2055. [DOD] [CrossRef]
- Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE, DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010. 376(9739):431-439. [DOD] [CrossRef]
- Raskin P, Mohan A. Comparison of once-weekly with twice-daily exenatide in the treatment of type 2 diabetes (DURATION-1 trial). Expert Opin Pharmacother 2010. 11(13):2269-2271. [DOD] [CrossRef]
- Reusch J, Stewart MW, Perkins CM, Cirkel DT, Ye J, Perry CR, Reinhardt RR, Bode BW. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes Obes Metab 2014. 16(12):1257-1264. [DOD] [CrossRef]
- Ratner RE, Maggs D, Nielsen LL, Stonehouse AH, Poon T, Zhang B, Bicsak TA, Brodows RG, Kim DD. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab 2006. 8(4):419-428. [DOD] [CrossRef]
- Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Maggs DG, Wintle ME. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007. 29(1):139-153. [DOD] [CrossRef]
- Exenatide SPC. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf. Accessed on 22.11.2014. [DOD]
- Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, Kuhstoss D, Lakshmanan M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014. 37(8):2159-2167. [DOD] [CrossRef]
- Sun F, Yu K, Yang Z, Wu S, Zhang Y, Shi L, Ji L, Zhan S. Impact of GLP-1 receptor agonists on major gastrointestinal disorders for type 2 diabetes mellitus: a mixed treatment comparison meta-analysis. Exp Diabetes Res 2012. 2012:230624. [DOD]
- Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L, Group D-S. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008. 372(9645):1240-1250. [DOD] [CrossRef]
- Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011. 60(5):1561-1565. [DOD] [CrossRef]
- Lee PH, Stockton MD, Franks AS. Acute pancreatitis associated with liraglutide. Ann Pharmacother 2011. 45(4):e22. [DOD] [CrossRef]
- Ayoub WA, Kumar AA, Naguib HS, Taylor HC. Exenatide-induced acute pancreatitis. Endocr Pract 2010. 16(1):80-83. [DOD] [CrossRef]
- Tripathy NR, Basha S, Jain R, Shetty S, Ramachandran A. Exenatide and acute pancreatitis. J Assoc Physicians India 2008. 56:987-988. [DOD]
- Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care 2006. 29(2):471. [DOD] [CrossRef]
- Jeyaraj S, Shetty AS, Kumar CR, Nanditha A, Krishnamoorthy S, Raghavan A, Raghavan K, Ramachandran A. Liraglutide-induced acute pancreatitis. J Assoc Physicians India 2014. 62(1):64-66. [DOD]
- Bourezane H, Kastler B, Kantelip JP. Late and severe acute necrotizing pancreatitis in a patient with liraglutide. Therapie 2012. 67(6):539-543. [DOD] [CrossRef]
- Nakata H, Sugitani S, Yamaji S, Otsu S, Higashi Y, Ohtomo Y, Inoue G. Pancreatitis with pancreatic tail swelling associated with incretin-based therapies detected radiologically in two cases of diabetic patients with end-stage renal disease. Intern Med 2012. 51(21):3045-3049. [DOD] [CrossRef]
- Famularo G, Gasbarrone L, Minisola G. Pancreatitis during treatment with liraglutide. JOP 2012. 13(5):540-541. [DOD]
- Knezevich E, Crnic T, Kershaw S, Drincic A. Liraglutide-associated acute pancreatitis. Am J Health Syst Pharm 2012. 69(5):386-389. [DOD] [CrossRef]
- Yu X, Tang H, Huang L, Yang Y, Tian B, Yu C. Exenatide-induced chronic damage of pancreatic tissue in rats. Pancreas 2012. 41(8):1235-1240. [DOD] [CrossRef]
- Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model. Diabetes 2012. 61(5):1250-1262. [DOD] [CrossRef]
- Rouse R, Zhang L, Shea K, Zhou H, Xu L, Stewart S, Rosenzweig B, Zhang J. Extended exenatide administration enhances lipid metabolism and exacerbates pancreatic injury in mice on a high fat, high carbohydrate diet. Plos One 2014. 9(10):e109477. [DOD] [CrossRef]
- Rouse R, Xu L, Stewart S, Zhang J. High fat diet and GLP-1 drugs induce pancreatic injury in mice. Toxicol Appl Pharmacol 2014. 276(2):104-114. [DOD] [CrossRef]
- Mondragon A, Davidsson D, Kyriakoudi S, Bertling A, Gomes-Faria R, Cohen P, Rothery S, Chabosseau P, Rutter GA, da Silva Xavier G. Divergent effects of liraglutide, exendin-4, and sitagliptin on beta-cell mass and indicators of pancreatitis in a mouse model of hyperglycaemia. Plos One 2014. 9(8):e104873. [DOD] [CrossRef]
- Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagon-like peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr Pract 2012. 18(4):472-477. [DOD] [CrossRef]
- Steinberg WM, Nauck MA, Zinman B, Daniels GH, Bergenstal RM, Mann JF, Steen Ravn L, Moses AC, Stockner M, Baeres FM, et al. LEADER 3-lipase and amylase activity in subjects with type 2 diabetes: baseline data from over 9000 subjects in the LEADER Trial. Pancreas 2014. 43(8):1223-1231. [DOD] [CrossRef]
- Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagon-like peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 2013. 173(7):534-539. [DOD] [CrossRef]
- Meier JJ, Nauck MA. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia 2014. 57(7):1320-1324. [DOD] [CrossRef]
- US Food and Drug Administration. http://www.fda.gov/Drugs/DrugSafety/ucm343187.htm. Accessed on 30.11.2014. [DOD]
- Li X, Zhang Z, Duke J. Glucagon-like peptide 1-based therapies and risk of pancreatitis: a self-controlled case series analysis. Pharmacoepidemiol Drug Saf 2014. 23(3):234-239. [DOD] [CrossRef]
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Glucagon-like peptide-1 receptor agonists and pancreatitis: a meta-analysis of randomized clinical trials. Diabetes Res Clin Pract 2014. 103(2):269-275. [DOD] [CrossRef]
- Faillie JL, Babai S, Crepin S, Bres V, Laroche ML, Le Louet H, Petit P, Montastruc JL, Hillaire-Buys D, French Pharmacovigilance Centers Network. Pancreatitis associated with the use of GLP-1 analogs and DPP-4 inhibitors: a case/non-case study from the French Pharmacovigilance Database. Acta Diabetol 2014. 51(3):491-497. [DOD]
- Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care 2010. 33(11):2349-2354. [DOD] [CrossRef]
- Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 2009. 25(4):1019-1027. [DOD] [CrossRef]
- Funch D, Gydesen H, Tornoe K, Major-Pedersen A, Chan KA. A prospective, claims-based assessment of the risk of pancreatitis and pancreatic cancer with liraglutide compared to other antidiabetic drugs. Diabetes Obes Metab 2014. 16(3):273-275. [DOD] [CrossRef]
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 2012. 98(2):271-284. [DOD] [CrossRef]
- Wenten M, Gaebler JA, Hussein M, Pelletier EM, Smith DB, Girase P, Noel RA, Braun DK, Bloomgren GL. Relative risk of acute pancreatitis in initiators of exenatide twice daily compared with other anti-diabetic medication: a follow-up study. Diabet Med 2012. 29(11):1412-1418. [DOD] [CrossRef]
- Dore DD, Bloomgren GL, Wenten M, Hoffman C, Clifford CR, Quinn SG, Braun DK, Noel RA, Seeger JD. A cohort study of acute pancreatitis in relation to exenatide use. Diabetes Obes Metab 2011. 13(6):559-566. [DOD] [CrossRef]
- Dore DD, Hussein M, Hoffman C, Pelletier EM, Smith DB, Seeger JD. A pooled analysis of exenatide use and risk of acute pancreatitis. Curr Med Res Opin 2013. 29(12):1577-1586. [DOD] [CrossRef]
- Giorda CB, Sacerdote C, Nada E, Marafetti L, Baldi I, Gnavi R. Incretin-based therapies and acute pancreatitis risk: a systematic review and meta-analysis of observational studies. Endocrine 2015. 48(2):461-471. [DOD] [CrossRef]
- Li L, Shen J, Bala MM, Busse JW, Ebrahim S, Vandvik PO, Rios LP, Malaga G, Wong E, Sohani Z, et al. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ 2014. 348:g2366. [DOD] [CrossRef]
- Wang T, Wang F, Gou Z, Tang H, Li C, Shi L, Zhai S. Using real-world data to evaluate the association of incretin-based therapies with risk of acute pancreatitis: a meta-analysis of 1,324,515 patients from observational studies. Diabetes Obes Metab 2015. 17(1):32-41. [DOD] [CrossRef]
- Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C. Pancreatic safety of incretin-based drugs-FDA and EMA assessment. N Engl J Med 2014. 370(9):794-797. [DOD] [CrossRef]
- Giorda CB, Nada E, Tartaglino B, Marafetti L, Gnavi R. A systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs: from hard facts to a balanced position. Diabetes Obes Metab 2014. 16(11):1041-1047. [DOD] [CrossRef]
- Scheen A. Gliptins (dipeptidyl peptidase-4 inhibitors) and risk of acute pancreatitis. Expert Opin Drug Saf 2013. 12(4):545-557. [DOD] [CrossRef]
- Filippatos TD, Elisaf MS. Recommendations for severe hypertriglyceridemia treatment, are there new strategies? Curr Vasc Pharmacol 2014. 12(4):598-616. [DOD]
- Ratner R, Han J, Nicewarner D, Yushmanova I, Hoogwerf BJ, Shen L. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol 2011. 10:22. [DOD] [CrossRef]
- Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, Hussein MA. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care 2011. 34(1):90-95. [DOD] [CrossRef]
- Schmidt LJ, Habacher W, Augustin T, Krahulec E, Semlitsch T. A systematic review and meta-analysis of the efficacy of lixisenatide in the treatment of patients with type 2 diabetes. Diabetes Obes Metab 2014. 16(9):769-779. [DOD] [CrossRef]
- Monami M, Cremasco F, Lamanna C, Colombi C, Desideri CM, Iacomelli I, Marchionni N, Mannucci E. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Exp Diabetes Res 2011. 2011:215764. [DOD]
- Katout M, Zhu H, Rutsky J, Shah P, Brook RD, Zhong J, Rajagopalan S. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens 2014. 27(1):130-139. [DOD] [CrossRef]
- Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013. 3(1):e001986. [DOD] [CrossRef]
- Ferdinand KC, White WB, Calhoun DA, Lonn EM, Sager PT, Brunelle R, Jiang HH, Threlkeld RJ, Robertson KE, Geiger MJ. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension 2014. 64(4):731-737. [DOD] [CrossRef]
- Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin-Mery A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab 2013. 15(7):642-649. [DOD] [CrossRef]
- Bohm M, Reil JC, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med 2015. 128(3):219-228. [DOD] [CrossRef]
- Chatterjee DJ, Khutoryansky N, Zdravkovic M, Sprenger CR, Litwin JS. Absence of QTc prolongation in a thorough QT study with subcutaneous liraglutide, a once-daily human GLP-1 analog for treatment of type 2 diabetes. J Clin Pharmacol 2009. 49(11):1353-1362. [DOD] [CrossRef]
- Linnebjerg H, Seger M, Kothare PA, Hunt T, Wolka AM, Mitchell MI. A thorough QT study to evaluate the effects of single dose exenatide 10 mug on cardiac repolarization in healthy subjects. Int J Clin Pharmacol Ther 2011. 49(10):594-604. [DOD] [CrossRef]
- Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, Braun D, Giaconia J, Malone J. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol 2010. 9:6. [DOD] [CrossRef]
- Darpo B, Zhou M, Matthews J, Zhi H, Young MA, Perry C, Reinhardt RR. Albiglutide does not prolong QTc interval in healthy subjects: A thorough ECG study. Diabetes Ther 2014. 5(1):141-153. [DOD] [CrossRef]
- Darpo B, Philip S, MacConell L, Cirincione B, Mitchell M, Han J, Huang W, Malloy J, Schulteis C, Shen L, Porter L. Exenatide at therapeutic and supratherapeutic concentrations does not prolong the QTc interval in healthy subjects. Br J Clin Pharmacol 2013. 75(4):979-989. [DOD] [CrossRef]
- Dulaglutide-EMA assessment report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002825/WC500179473.pdf. Accessed on 18.4.2015. [DOD]
- Lixisenatide SPC. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Accessed on 22.11.2014. [DOD]
- Liraglutide SPC. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. Accessed on 22.11.2014. [DOD]
- Albiglutide SPC. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf. Accessed on 22.11.2014. [DOD]
- Buse JB, Garber A, Rosenstock J, Schmidt WE, Brett JH, Videbaek N, Holst J, Nauck M. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. J Clin Endocrinol Metab 2011. 96(6):1695-1702. [DOD] [CrossRef]
- Faludi P, Brodows R, Burger J, Ivanyi T, Braun DK. The effect of exenatide re-exposure on safety and efficacy. Peptides 2009. 30(9):1771-1774. [DOD] [CrossRef]
- Fineman MS, Mace KF, Diamant M, Darsow T, Cirincione BB, Booker Porter TK, Kinninger LA, Trautmann ME. Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab 2012. 14(6):546-554. [DOD] [CrossRef]
- Exenatide once weekly SPC. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf. Accessed on 22.11.2014. [DOD]
- Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists - available efficacy and safety data and perspectives for the future. Diabetes Obes Metab 2011. 13(5):394-407. [DOD] [CrossRef]
- Shan SJ, Guo Y. Exenatide-induced eosinophilic sclerosing lipogranuloma at the injection site. Am J Dermatopathol 2014. 36(6):510-512. [DOD] [CrossRef]
- Boysen NC, Stone MS. Eosinophil-rich granulomatous panniculitis caused by exenatide injection. J Cutan Pathol 2014. 41(1):63-65. [DOD] [CrossRef]
- Andres-Ramos I, Blanco-Barrios S, Fernandez-Lopez E, Santos-Briz A. Exenatide-induced eosinophil-rich granulomatous panniculitis: A novel case showing injected microspheres. Am J Dermatopathol 2014. In press. [DOD]
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005. 28(5):1092-1100. [DOD] [CrossRef]
- Zinman B, Hoogwerf BJ, Duran Garcia S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007. 146(7):477-485. [DOD] [CrossRef]
- Norwood P, Liutkus JF, Haber H, Pintilei E, Boardman MK, Trautmann ME. Safety of exenatide once weekly in patients with type 2 diabetes mellitus treated with a thiazolidinedione alone or in combination with metformin for 2 years. Clin Ther 2012. 34(10):2082-2090. [DOD] [CrossRef]
- Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L, LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009. 32(7):1224-1230. [DOD] [CrossRef]
- Ratner RE, Rosenstock J, Boka G. Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 2010. 27(9):1024-1032. [DOD] [CrossRef]
- Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metab 2013. 15(11):1000-1007. [DOD] [CrossRef]
- Home PD, Shamanna P, Stewart M, Yang F, Miller M, Perry C, Carr MC. Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes Obes Metab 2015. 17(2):179-187. [DOD] [CrossRef]
- Ahren B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, Feinglos MN, HARMONY-3 Study Group. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014. 37(8):2141-2148. [DOD] [CrossRef]
- Pencek R, Brunell SC, Li Y, Hoogwerf BJ, Malone J. Exenatide once weekly for the treatment of type 2 diabetes mellitus: clinical results in subgroups of patients using different concomitant medications. Postgrad Med 2012. 124(4):33-40. [DOD] [CrossRef]
- Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005. 28(5):1083-1091. [DOD] [CrossRef]
- Gao Y, Yoon KH, Chuang LM, Mohan V, Ning G, Shah S, Jang HC, Wu TJ, Johns D, Northrup J, Brodows R. Efficacy and safety of exenatide in patients of Asian descent with type 2 diabetes inadequately controlled with metformin or metformin and a sulphonylurea. Diabetes Res Clin Pract 2009. 83(1):69-76. [DOD] [CrossRef]
- Kaku K, Rasmussen MF, Clauson P, Seino Y. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human glucagon-like peptide-1 analogue liraglutide as add-on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2010. 12(4):341-347. [DOD] [CrossRef]
- Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012. 14(10):910-917. [DOD] [CrossRef]
- Levin P, Wei W, Wang L, Pan C, Douglas D, Baser O. Combination therapy with insulin glargine and exenatide: real-world outcomes in patients with type 2 diabetes. Curr Med Res Opin 2012. 28(3):439-446. [DOD] [CrossRef]
- Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract 2012. 18(1):17-25. [DOD] [CrossRef]
- Grimm M, Han J, Weaver C, Griffin P, Schulteis CT, Dong H, Malloy J. Efficacy, safety, and tolerability of exenatide once weekly in patients with type 2 diabetes mellitus: an integrated analysis of the DURATION trials. Postgrad Med 2013. 125(3):47-57. [DOD] [CrossRef]
- Ivanyi T, Fovenyi J, Faludi P, Han J, Macconell L, Wille S, Kiljanski J. Long-term effects of adding exenatide to a regimen of metformin and/or sulfonylurea in type 2 diabetes: an uncontrolled, open-label trial in Hungary. Clin Ther 2012. 34(6):1301-1313. [DOD] [CrossRef]
- DeVries JH, Bain SC, Rodbard HW, Seufert J, D'Alessio D, Thomsen AB, Zychma M, Rosenstock J, Liraglutide-Detemir Study Group. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1c targets. Diabetes Care 2012. 35(7):1446-1454. [DOD] [CrossRef]
- Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, Ping L, Rosenstock J. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care 2013. 36(9):2497-2503. [DOD] [CrossRef]
- Seino Y, Min KW, Niemoeller E, Takami A, EFC10887 GETGOAL-L ASIA Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012. 14(10):910-917. [DOD] [CrossRef]
- Thong KY, Jose B, Sukumar N, Cull ML, Mills AP, Sathyapalan T, Shafiq W, Rigby AS, Walton C, Ryder RE. Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Diabetologists nationwide exenatide audit. Diabetes Obes Metab 2011. 13(8):703-710. [DOD] [CrossRef]
- de Heer J, Holst JJ. Sulfonylurea compounds uncouple the glucose dependence of the insulinotropic effect of glucagon-like peptide 1. Diabetes 2007. 56(2):438-443. [DOD] [CrossRef]
- Li CJ, Li J, Zhang QM, Lv L, Chen R, Lv CF, Yu P, Yu DM. Efficacy and safety comparison between liraglutide as add-on therapy to insulin and insulin dose-increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol 2012. 11:142. [DOD] [CrossRef]
- Su B, Sheng H, Zhang M, Bu L, Yang P, Li L, Li F, Sheng C, Han Y, Qu S, Wang J. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists' treatment: a meta-analysis of randomized controlled trials. Endocrine 2015. 48(1):107-115. [DOD] [CrossRef]
- Bunck MC, Eliasson B, Corner A, Heine RJ, Shaginian RM, Taskinen MR, Yki-Jarvinen H, Smith U, Diamant M. Exenatide treatment did not affect bone mineral density despite body weight reduction in patients with type 2 diabetes. Diabetes Obes Metab 2011. 13(4):374-377. [DOD] [CrossRef]
- Filippatos TD, Elisaf MS. Effects of glucagon-like peptide-1 receptor agonists on renal function. World J Diabetes 2013. 4(5):190-201. [DOD]
- Johansen OE, Whitfield R. Exenatide may aggravate moderate diabetic renal impairment: a case report. Br J Clin Pharmacol 2008. 66(4):568-569. [DOD] [CrossRef]
- Ferrer-Garcia JC, Martinez-Chanza N, Tolosa-Torrens M, Sanchez-Juan C. Exenatide and renal failure. Diabet Med 2010. 27(6):728-729. [DOD] [CrossRef]
- Lopez-Ruiz A, del Peso-Gilsanz C, Meoro-Aviles A, Soriano-Palao J, Andreu A, Cabezuelo J, Arias JL. Acute renal failure when exenatide is co-administered with diuretics and angiotensin II blockers. Pharm World Sci 2010. 32(5):559-561. [DOD] [CrossRef]
- Dubois-Laforgue D, Boutboul D, Levy DJ, Joly D, Timsit J. Severe acute renal failure in patients treated with glucagon-like peptide-1 receptor agonists. Diabetes Res Clin Pract 2014. 103(3):e53-e55. [DOD] [CrossRef]
- Weise WJ, Sivanandy MS, Block CA, Comi RJ. Exenatide-associated ischemic renal failure. Diabetes Care 2009. 32(2):e22-23. [DOD] [CrossRef]
- US Food and Drug Administration. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm188656.htm. Accessed on 31.11.2014. [DOD]
- Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 2004. 89(6):3055-3061. [DOD] [CrossRef]
- Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, Gutmann H, Stanga Z, Vogel D, Beglinger C. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion 2006. 73(2-3):142-150. [DOD] [CrossRef]
- Kei A, Liberopoulos E, Siamopoulos K, Elisaf M. A patient with exenatide-associated acute-on-chronic renal failure requiring hemodialysis. Cardiovasc Contin 2011. 2:14-16. [DOD]
- Nandakoban H, Furlong TJ, Flack JR. Acute tubulointerstitial nephritis following treatment with exenatide. Diabet Med 2013. 30(1):123-125. [DOD] [CrossRef]
- Bhatti R, Flynn MD, Illahi M, Carnegie AM. Exenatide associated renal failure. Pract Diabetes Int 2010. 27(6):232-234. [DOD] [CrossRef]
- Macconell L, Brown C, Gurney K, Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes 2012. 5:29-41. [DOD]
- Pendergrass M, Fenton C, Haffner SM, Chen W. Exenatide and sitagliptin are not associated with increased risk of acute renal failure: a retrospective claims analysis. Diabetes Obes Metab 2012. 14(7):596-600. [DOD] [CrossRef]
- Narayana SK, Talab SK, Elrishi MA. Liraglutide-induced acute kidney injury. Pract Diabetes 2012. 29(9):380-382. [DOD] [CrossRef]
- Kaakeh Y, Kanjee S, Boone K, Sutton J. Liraglutide-induced acute kidney injury. Pharmacotherapy 2012. 32(1):e7-e11. [DOD] [CrossRef]
- Gariani K, de Seigneux S, Moll S. Acute interstitial nephritis after treatment with liraglutide. Am J Kidney Dis 2014. 63(2):347. [DOD] [CrossRef]
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care 2013. 36(7):2118-2125. [DOD]
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013. 36(7):2126-2132. [DOD] [CrossRef]
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013. 62(7):2595-2604. [DOD] [CrossRef]
- Tseng CC, Zhang XY, Wolfe MM. Effect of GIP and GLP-1 antagonists on insulin release in the rat. Am J Physiol 1999. 276(6 Pt 1):E1049-E1054. [DOD]
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011. 141(1):150-156. [DOD] [CrossRef]
- Feng X, Cai A, Dong K, Chaing W, Feng M, Bhutada NS, Inciardi J, Woldemariam T. Assessing pancreatic cancer risk associated with dipeptidyl peptidase 4 inhibitors: Data mining of FDA Adverse Event Reporting System (FAERS). J Pharmacovigilance 2013. 1(3):110. [DOD]
- Bonner-Weir S, In't Veld PA, Weir GC. Reanalysis of study of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab 2014. 16(7):661-666. [DOD] [CrossRef]
- Gotfredsen CF, Molck AM, Thorup I, Nyborg NC, Salanti Z, Knudsen LB, Larsen MO. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes 2014. 63(7):2486-2497. [DOD] [CrossRef]
- Nyborg NC, Molck AM, Madsen LW, Knudsen LB. The human GLP-1 analog liraglutide and the pancreas: evidence for the absence of structural pancreatic changes in three species. Diabetes 2012. 61(5):1243-1249. [DOD] [CrossRef]
- Romley JA, Goldman DP, Solomon M, McFadden D, Peters AL. Exenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured population. Diabetes Technol Ther 2012. 14(10):904-911. [DOD] [CrossRef]
- Dore DD, Seeger JD, Chan KA. Incidence of health insurance claims for thyroid neoplasm and pancreatic malignancy in association with exenatide: signal refinement using active safety surveillance. Ther Adv Drug Saf 2012. 3(4):157-164. [DOD] [CrossRef]
- Zhao H, Wang L, Wei R, Xiu D, Tao M, Ke J, Liu Y, Yang J, Hong T. Activation of glucagon-like peptide-1 receptor inhibits tumourigenicity and metastasis of human pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes Metab 2014. 16(9):850-860. [DOD] [CrossRef]
- Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010. 151(4):1473-1486. [DOD] [CrossRef]
- Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjogren I, Andersen S, Andersen L, de Boer AS, Manova K, Barlas A, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 2012. 153(3):1538-1547. [DOD] [CrossRef]
- Vahle JL, Byrd RA, Blackbourne JL, Martin JA, Sorden SD, Ryan T, Pienkowski T, Wijsman JA, Smith HW, Rosol TJ. Effects of Dulaglutide on Thyroid C-Cells and Serum Calcitonin in Male Monkeys. Endocrinology 2015. 2015:en20141717. [DOD]
- Rosol TJ. On-target effects of GLP-1 receptor agonists on thyroid C-cells in rats and mice. Toxicol Pathol 2013. 41(2):303-309. [DOD] [CrossRef]
- Hegedus L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011. 96(3):853-860. [DOD] [CrossRef]
- Bode SF, Egg M, Wallesch C, Hermanns-Clausen M. 10-fold liraglutide overdose over 7 months resulted only in minor side-effects. J Clin Pharmacol 2013. 53(7):785-786. [DOD] [CrossRef]
- Nakanishi R, Hirose T, Tamura Y, Fujitani Y, Watada H. Attempted suicide with liraglutide overdose did not induce hypoglycemia. Diabetes Res Clin Pract 2013. 99(1):e3-e4. [DOD] [CrossRef]
- Truitt C, Brooks D, Skolnik A. Largest reported liraglutide overdose. North American Congress of Clinical Toxicology 2011. Abstract 112. Available from: http://www.clintox.org/NACCT/2011/NACCT2011abstractsAllPoster.pdf. Accessed on 23.11.14. [DOD]
- Cohen V, Teperikidis E, Jellinek SP, Rose J. Acute exenatide (Byetta) poisoning was not associated with significant hypoglycemia. Clin Toxicol (Phila) 2008. 46(4):346-347. [DOD] [CrossRef]
- Krishnan L, Dhatariya K, Gerontitis D. No clinical harm from a massive exenatide overdose: a short report. Clin Toxicol (Phila) 2013. 51(1):61. [DOD] [CrossRef]
- Elmehdawi RR, Elbarsha AM. An accidental liraglutide overdose: case report. Libyan J Med 2014. 9:23055. [DOD] [CrossRef]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004. 27(11):2628-2635. [DOD] [CrossRef]
- Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008. 372(9645):1240-1250. [DOD] [CrossRef]
- Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, Wilhelm K, Trautmann M, Shen LZ, Porter LE, DURATION-1 Study Group. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010. 33(6):1255-1261. [DOD] [CrossRef]
- Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, Trautmann M, Porter L. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011. 96(5):1301-1310. [DOD] [CrossRef]
- Rosenstock J, Raccah D, Koranyi L, Maffei L, Boka G, Miossec P, Gerich JE. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 2013. 36(10):2945-2951. [DOD] [CrossRef]
- Diamant M, Van Gaal L, Guerci B, Stranks S, Han J, Malloy J, Boardman MK, Trautmann ME. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol 2014. 2(6):464-473. [DOD] [CrossRef]
- Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Thomsen AB, Sondergaard RE, Davies M. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010. 375(9724):1447-1456. [DOD] [CrossRef]
- Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B, LEAD-3 (Mono) Study Group. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab 2011. 13(4):348-356. [DOD] [CrossRef]
- Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Garber A, Thomsen AB, Hartvig H, Davies M, 1860 LIRA-DPP-4 Study Group. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract 2011. 65(4):397-407. [DOD] [CrossRef]
- Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012. 36(6):843-854. [DOD] [CrossRef]
- Dungan KM, Povedano ST, Forst T, Gonzalez JG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 2014. 384(9951):1349-1357. [DOD] [CrossRef]
- Ahren B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 2013. 36(9):2543-2550. [DOD] [CrossRef]
- Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, Hanefeld M. Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med 2014. 31(2):176-184. [DOD] [CrossRef]
- Meier JJ, Rosenstock J, Hincelin-Mery A, Roy-Duval C, Delfolie A, Coester HV, Menge BA, Forst T, Kapitza C. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: A randomized, open-label trial. Diabetes Care 2015. In press. [DOD]
- Rosenstock J, Fonseca VA, Gross JL, Ratner RE, Ahren B, Chow FC, Yang F, Miller D, Johnson SL, Stewart MW, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care 2014. 37(8):2317-2325. [DOD] [CrossRef]
- Leiter LA, Carr MC, Stewart M, Jones-Leone A, Scott R, Yang F, Handelsman Y. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care 2014. 37(10):2723-2730. [DOD] [CrossRef]
- Weissman PN, Carr MC, Ye J, Cirkel DT, Stewart M, Perry C, Pratley R. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia 2014. 57(12):2475-2484. [DOD] [CrossRef]
- Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care 2015. 37(8):2149-2158. [DOD] [CrossRef]
- Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014. 37(8):2168-2176. [DOD] [CrossRef]
- Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab 2015. In press. [DOD]
- Davies M, Heller S, Sreenan S, Sapin H, Adetunji O, Tahbaz A, Vora J. Once-weekly exenatide versus once- or twice-daily insulin detemir: randomized, open-label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care 2013. 36(5):1368-1376. [DOD] [CrossRef]
- Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, Zhou T, Muehlen-Bartmer I, Ratner RE. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J Diabetes Complications 2014. 28(3):386-392. [DOD] [CrossRef]
This article has been cited by other articles:

|
Efficacy and risk profile of anti-diabetic therapies: Conventional vs traditional drugs-A mechanistic revisit to understand their mode of action
Gupta P, Bala M, Gupta S, Dua A, Dabur R, Injeti E, Mittal A
Pharmacol Res 2016. 113(Pt A):636-674
|
|

|
Albiglutide-induced pancreatitis
Jain N, Savani M, Agarwal M, Sands CW
Ther Adv Drug Saf 2016. 7(6):236-238
|
|

|
GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial
Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A, Filippaios A, Bowers J, Srnka A, Gavrieli A, Ko BJ, Liakou C, Kanyuch N, Tseleni-Balafouta S, Mantzoros CS
Diabetologia 2016. 59(5):954-965
|
|
|
Diabetes and Pancreas: Why So Difficult? Potential Mechanisms of Elevated Serum Pancreatic Enzymes
Matteucci E, Giampietro O
Curr Med Chem 2016. 23(3):290-302
|
|
|