Original Data
| Rev Diabet Stud,
2017,
14(4):372-380 |
DOI 10.1900/RDS.2017.14.372 |
Duration of Type 2 Diabetes is a Predictor of Elevated Plantar Foot Pressure
Brooke Falzon1, Cynthia Formosa1, Liberato Camilleri2, Alfred Gatt1
1Department of Podiatry, Faculty of Health Sciences, University of Malta, Msida, MSD 2080, Malta
2Department of Statistics and Operations Research, Faculty of Science, University of Malta, Msida, MSD 2080, Malta
Address correspondence to: Alfred Gatt, e-mail: alfred.gatt@um.edu.mt
Manuscript submitted September 11, 2017; resubmitted November 23, 2017; accepted January 22, 2018.
Keywords: diabetes, foot, diabetes complication, peak plantar pressure, pressure-time integral, metatarsophalangeal joint, foot posture index, ulceration
Abstract
AIMS: Elevated plantar pressure is considered a significant risk factor for ulceration in diabetes mellitus. The aim of this study was to determine whether duration of diabetes could affect plantar pressure in patients with no known significant comorbidity or foot pathology. METHODS: Participants with type 2 diabetes, but without known confounding factors that could alter peak pressure, were matched for age, weight, and gender and categorized into 3 groups of diabetes duration: group 1 (1-5 yr), group 2 (6-10 yr), and group 3 (11-15 yr). Plantar pressures were recorded utilizing a two-step protocol at a self-selected speed. RESULTS: One-way analysis of variance (ANOVA) revealed significant differences in mean peak plantar pressures between the three groups under the 2nd - 4th metatarsophalangeal joint (MPJ) region of interest (ROI) (p = 0.012 and p = 0.022, respectively) and left heel (p = 0.049). Also, a significant difference in mean pressure-time integral under the left 2nd - 4th MPJ ROI (p = 0.021) and right heel (p = 0.048) was observed. Regression analysis confirmed that mean peak plantar pressures in the first group (but not in the second group) were significantly lower than in the third group (p = 0.005). CONCLUSIONS: As the duration of diabetes increased, peak plantar pressure increased significantly under the 2nd - 4th MPJ ROIs. These findings suggest that clinicians should make more use of pressure mapping technology as part of their clinical management plan in patients with diabetes >10 yr, even if they have no complications or deformities, to preserve functional limbs in this high-risk population.
Abbreviations: ADA - American Diabetes Association; ANOVA - analysis of variance; FPI - foot posture index; IDF - International Diabetes Federation; kPa - kilopascal; kPa/s/cm2 - kilopascal/sec/cm2; MPJ - metatarsophalangeal joint; NICE - National Institute for Health and Care Excellence; PTI - pressure-time integral; ROI - region of interest; SIGN - Scottish Intercollegiate Guidelines Network; T2D - type 2 diabetes
1. Introduction
Diabetes mellitus may cause several complications. Foot ulceration and lower limb amputations are among the most severe and common complications [1]. People living with diabetes carry a risk of foot ulceration ranging from 4-10%, with a lifetime risk of up to 25% [2]. Nearly 85% of all amputations in people with diabetes are preceded by ulceration. Since a large percentage of diabetes-related lower limb amputations are potentially avoidable, it is important to implement effective and practicable diabetic foot care protocols in primary care settings to prevent these complications [3].
Limited joint mobility [4, 5], structural foot deformities [6], intrinsic muscle weakness [7], and muscle atrophy [8] occur in both type 1 and type 2 diabetes. These complications may occur as a result of hyperglycemia and play a significant role in increasing plantar pressures [9].
Plantar pressure measurement is frequently used to assess gait conditions associated with diabetes as excessive pressure in specific areas may cause foot ulceration in patients with diabetes. Abnormal patterns of foot loading in these patients caused by abnormal gait that has developed over time may lead to various foot complications such as ulcers. Changes in foot pressures can be determined by assessing peak plantar pressures or pressure-time integral [5]. Although Anjos et al. reported that patients living with diabetes for more than 10 years may have increased peak plantar pressure [9], which is also supported by Tuna et al. [10], there is a clear paucity of information regarding this matter.
Alterations in the distribution of plantar pressure may be due to several factors, including changes in the anatomical structure of the foot and deformities [11]. When subjects with diabetes and a history of ulceration were compared with those without ulceration or healthy individuals, higher plantar pressures were noted on the lateral side of the forefoot area [12].
The majority of previous studies evaluated the roles of established peripheral neuropathy [13, 14] and peripheral vascular disease [15] as major causes of plantar pressure abnormalities. Few studies have examined plantar pressure distribution in patients with type 2 diabetes (T2D) without neuropathy [16]. It has been suggested that foot pressure abnormality may represent an early marker of diabetic peripheral neuropathy.
A peak plantar pressure threshold higher than 6 kg/cm2 (588.6 kPa) during walking has been hypothesized as the pressure threshold that can cause soft tissue damage in high-risk patients with T2D [17]. Another study reported that the peak plantar pressure threshold in patients with T2D and hammer or claw toe deformation measured 621 kPa under the second and third metatarsal head [18]. This increase in plantar pressure is attributed to a distal displacement of the protective fat pad under the metatarsophalangeal joints, such that plantar pressure is transferred proximally under the metatarsal heads. This reduced plantar tissue thickness is associated with higher peak plantar pressures, indicating greater risk of forefoot ulceration [6]. Continuous pulling on the metatarsal heads and thickening of the plantar fascia and Achilles tendon together with neuropathy may further increase pressure in the forefoot [19]. On the other hand, Fawzy et al. report that peak plantar pressure threshold before ulceration may be as low as 335 kPa [26].
Screening for diabetes involves the identification of asymptomatic individuals who are at high risk of developing the disease or its complications through appropriate screening tests [20]. Hence, the investigation of peak plantar pressure alterations in the non-symptomatic diabetic foot may be an important procedure for avoiding foot complications in high-risk individuals. This consideration has prompted this study, the aim of which is to investigate the relationship between duration of diabetes and plantar pressure in the absence of significant deformity or neuropathy.
2. Methods
2.1 Participants
The participants were recruited from a diabetes primary care outpatient clinic. Ethical approval was obtained from the University Research Ethics Committee, and all participants gave informed consent prior to inclusion in the study to meet the World Medical Association (2013) standards.
Thirty-six participants (61% male, 39% female) without foot deformities (i.e. with a neutral foot posture index (FPI) score of 0 to +5) were recruited. The participants were divided into 3 groups according to disease duration matched for age, gender, and body weight, as follows:
- Group 1: 12 participants who had lived with T2D for the past 5 years.
- Group 2: 12 participants who had lived with T2D for 6 to 10 years.
- Group 3: 12 participants who had lived with T2D for 11 to 15 years.
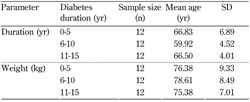
The participant age and weight parameters are shown in Table 1.
Table
1.
Age and weight parameters of participants in the three diabetes duration groups |
|
 |
 |
3.2 Assessment of neuropathy
All participants were initially assessed for the presence of sensory peripheral neuropathy by 5.07 Semmes-Weinstein monofilament and vibration perception threshold using a tuning fork. The monofilament was applied to the skin of the feet while the participants' eyes were closed. The monofilament was applied at 10 sites on each foot. Any negative response to the monofilament or tuning fork that indicated the possible presence of neuropathy resulted in the exclusion of this patient from the study.
3.3 Assessment of range of motion
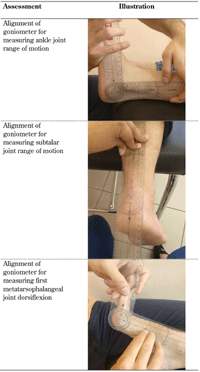
Participants were then assessed for range of motion in the major joints while on a couch in a supine position with the use of a goniometer. Range of motion testing for the ankle, and the subtalar and first metatarsophalangeal joint is shown in Table 2. All biomechanical examinations were carried out according to accepted clinical practice, and detection of biomechanical or structural deformity resulted in the exclusion of the participant from the study [21].
Table
2.
Biomechanical assessment according to Gastwirth, 1996 [21] |
|
 |
 |
3.4 Foot posture assessment
Foot posture was assessed using the foot posture index, which is a validated tool for determining whether the foot is neutral, supinated, or pronated [22]. Only those participants with a neutral foot posture (0 to +5) were included, since both supinated and pronated feet have been shown to alter plantar pressure.
3.5 Foot plantar pressure assessment
Each participant was then asked to walk on a Tekscan (Boston, USA) HR Mat™ using the two-step gait protocol, and the participants' dynamic plantar pressures were recorded. This high-resolution pressure mapping platform (4.1 sensels/cm2) has been validated previously and utilized in a number of similar research projects [23]. All data were recorded at 55 Hz. Three trials were recorded for each participant while they were walking at the participant's own preferred pace and looking straight ahead [24], since this procedure was previously found to be sufficient to ensure that plantar pressure and force measurements were reliable [25]. Trials were excluded and repeated if the participant made one of the following mistakes:
- Misplacement of the foot on the pressure mat
- Discontinuation or cancellation of the walk on the mat for more than two steps
Standardized instructions were given to participants by the same researcher to ensure uniformity.
Each trial included eight stance phases: four stance phases for the left foot and four for the right foot. Before analyzing plantar pressure data, each participant’s first and last step was discarded as these could have biased the results regarding mean values. This procedure was applied as the initial step may not be representative of the patient’s gait style, while the recording of the last step could suffer from recording errors. All pressure recordings were performed by using Research FootMat™ Software Version 7. We also applied masks to mark the areas of interest on the following five foot regions:
- Hallux
- 1st metatarsophalangeal joint (MPJ)
- 2nd to 4th metatarsophalangeal joints
- 5th metatarsophalangeal joint and heel
- Heel
For each compartment, the mean reading of the three trials of peak plantar pressure measured in kPa, pressure time integral (PTI) measured in kPa/cm2, and contact area (cm2) were recorded. This process was repeated for the three subject groups.
3.6 Statistical analysis
We used the Shapiro Wilk test to test normality of the distribution of data regarding PTI and peak plantar pressure. Given normality, we compared mean peak plantar pressure at each foot mask between the three groups of diabetes duration (0-5, 6-10, 11-15 years) by using one-way analysis of variance (ANOVA).
A two-way ANOVA regression model was fitted to relate peak plantar pressures of the 2nd - 4th MPJ to two categorical predictors (diabetes duration, foot orientation, and their interaction). The advantage of using statistical modeling is that predictors can be analyzed collectively rather than individually.
3. Results
3.1 Comparison analysis
Figure 1 illustrates the mean peak plantar pressure of each foot mask for both the left and right foot reported by diabetes duration group. It can be seen that there was an increase in both the left and right 2nd - 4th MPJ region of interest (ROI) mean peak plantar pressure in participants from all three groups. The mean peak plantar pressure was also observed to increase gradually as the duration of diabetes increases, most significantly in the 2nd group (6-10 years) and in the 3rd group (11-15 years).
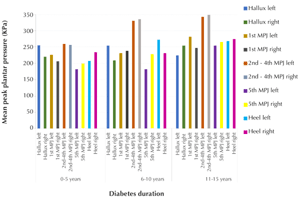 |
 |
Figure 1. Mean peak plantar pressure. The figure shows the mean peak plantar pressures for each duration of diabetes (i.e., 0-5 years, 6-10 years, and 11-15 years) and for each region of interest, as outlined in the column at the right, i.e. hallux, 1st metatarsophalangeal joint (MPJ), 2nd - 4th MPJ, and 5th MPJ for both feet. |
|
Figure 2 shows the mean PTI of each foot mask for both the left and right foot reported by diabetes duration group. The figure shows that there was an increase in both the left and right 2nd - 4th MPJ ROI mean pressure-time integral in participants in all three groups.
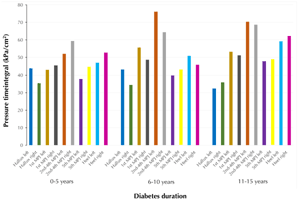 |
 |
Figure 2. Mean pressure-time integral (PTI). The figure shows the PTIs for each duration of diabetes (i.e., 0-5 years, 6-10 years, and 11-15 years) and for each region of interest, as outlined in the column at the right, i.e. hallux, 1st metatarsophalangeal joint (MPJ), 2nd - 4th MPJ, and 5th MPJ for both feet. |
|
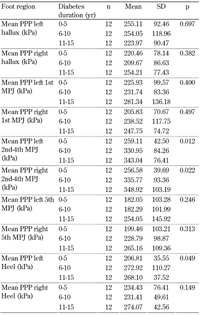
Comparison of PTI and peak plantar pressure variables between the three groups, i.e. in relation to duration of diabetes, showed that mean peak plantar pressure values increased gradually as the duration of diabetes increased except for hallux, left 1st MPJ, and both heels (Table 3). All mean peak plantar pressure values increased when comparing group 1 to group 3 except for the left hallux.
Table
3.
One-way ANOVA test results for mean peak plantar pressure at all foot regions between the three independent groups clustered by diabetes duration (0-5, 6-10, 11-15 years) |
|
 |
 |
Legend:
kPa - kilopascal, PPP - peak plantar pressure, SD - standard deviation. |
|
3.2 Regression analysis
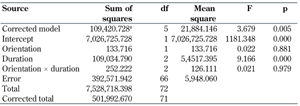
Regression analysis was used to relate peak plantar pressure of the 2nd - 4th MPJ to two predictors, namely duration of diabetes and foot orientation (left or right). The test of between-subject effects (Table 4) was used to identify significant predictors of peak plantar pressure of the 2nd - 4th MPJ. The two-predictor model identified diabetes duration as the sole significant predictor (p < 0.05). Foot orientation and the interaction effect were not found to be significant. This two-predictor model explained 21.8% of the total variation in peak plantar pressure in the 2nd - 4th MPJ foot region (r2 = 0.218) (Table 4).
Table
4.
Tests of between-subjects effects |
|
 |
 |
Legend:
a r2 = 0.218, the measure of the model goodness of fit. F: parameter mean square/error mean square. If the F-value increases the p-value decreases. |
|
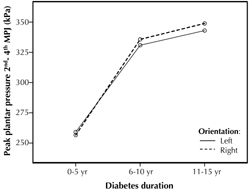
The graphs in Figure 3 illustrate the results of the regression model. They show that mean peak plantar pressure varies considerably between the 3 diabetes duration groups, in particular between the first two groups. There is also little variation in mean peak plantar pressure between the left and right foot, and the lines are fairly parallel, explaining why the interaction effect was not found to be significant.
 |
 |
Figure 3. Two-predictor model relating mean peak plantar pressure to duration of diabetes and foot orientation. The results of the regression model showed that duration of diabetes, but not foot orientation (i.e. left or right) is a significant predictor of peak plantar pressures. |
|
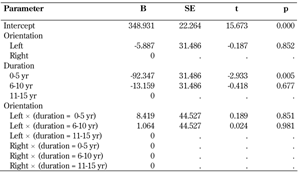
Table 5 shows the parameter estimates, indicating how much peak plantar pressure of the 2nd - 4th MPJ varies between the categories of diabetes duration and foot orientation (i.e., left or right). The regression coefficient (parameter estimate) for duration 0-5 years was -92.347, i.e. the mean peak plantar pressure for the first group was 92.347 KPa less on average than the third group, which was statistically significant (p = 0.005). The intercept, as reported in Table 5, is the expected peak plantar pressure of the 2nd - 4th MPJ; this is the expected peak plantar pressure of the 2nd - 4th MPJ for patients whose diabetes duration is 10-15 years.
Table
5.
Parameter estimates |
|
 |
 |
Legend:
Intercept - expected peak plantar pressure of the 2nd - 4th MPJ, B - regression coefficient of each parameter, SE - standard error, t - B/SE (if the t-value increases the p-value decreases). |
|
The coefficient for duration 6-10 years did not show statistical significance. All other estimates were also insignificant.
4. Discussion
This study reports the quantification of peak plantar pressure and PTI in participants with different duration of diabetes and no signs of foot deformities. Surprisingly, even though there were no obvious reasons for increased pressure in patients from the >10-year duration group, such as foot or toe deformity, they exhibited a mean peak plantar pressure of 348 kPa, which exceeds the lowest pressure threshold quoted that may cause tissue breakdown [26]. Should any of these participants develop peripheral arterial disease, neuropathy, or any foot deformity, their risk of ulceration would increase instantly.
The mechanism for the formation of this elevated latent peak plantar pressure and PTI in the absence of deformity and other obvious confounding factors may be attributable to non-enzymatic glycosylation of structural proteins and glycoproteins of the plantar soft tissues and overlying skin, which alters tissue stiffness and decreases skin flexibility. Injury in the diabetic foot is likely to initiate ulceration in the deep tissue layers, not on the skin surface, with the tissues underlying the distal bony prominences of the metatarsals being the most susceptible to tissue injury [27]. Skin and tissue breakdown may be caused by:
- Reduced flexibility
- Skin manifestations such as xerosis, cracks, or fissures due to altered function of sweat glands or damage to autonomic nerve fibers [28]
- Disruption in subcutaneous tissues
- Micro-hemorrhages
The combination of two or more of these processes may result in the formation of ulcers [29]. By the time ulceration becomes evident, damage to the underlying structures may already be exten the underlying structures may already be extensive.
Therefore, clinicians should do their utmost actually to prevent ulceration through patient education, appropriate screening techniques, and, most importantly, through the correct clinical biomechanical investigations which are known to be valid and reliable. Unfortunately, most of the time, patients are only assessed and treated when some form of pathology is already present.
Although high-peak plantar pressure is known to be an important risk factor, there is still little evidence regarding the causative mechanism of ulceration. It is hypothesized that high plantar pressure causes local ischemia in the underlying tissues, which consequently results in ulceration. The actual threshold of peak plantar pressure before ulceration occurs has been postulated to be as low as 335 kPa [26], which is lower than the mean pressure of 348.93 kPa, as reported in the present study. This result suggests the following alternative conclusions:
- If 335 kPa is indeed the correct threshold value before ulceration, "healthy" diabetes patients may exhibit peak plantar pressures that are dangerously close to or above this point, and the only reason why they have not ulcerated may be that they do not have neuropathy or peripheral arterial disease.
- 335 kPa is simply too low to serve as a cutoff value. Higher values may be more realistic [17].
Screening guidelines are known to be important for the prevention of foot ulceration and amputation. However, it is also evident that there is a distinct lack of scientific evidence regarding the various screening criteria [20]. Foot pressure measuring is a case in point, with the potential to become a screening guideline since, at the moment, there is absolutely no reference to foot pressure assessment as a means of preventing foot ulceration [30]. The authors thus recommend screening for high-peak pressure areas in order to assess the risk of ulceration before the actual soft tissue damage occurs.
5. Limitations
A limitation of the study is the small sample size. However, statistical significance was achieved with the number of participants included, confirming that the findings cannot be attributed to chance. Therefore, these results may be considered as preliminary findings which should encourage further research in the field to increase the level of evidence for the importance of measuring peak plantar pressures in this high-risk population. Because of the short duration of the study we are also unable to make a statement regarding longitudinal development, i.e. we do not know whether these individuals actually do ulcerate. Therefore, a longitudinal follow-up study would be necessary to confirm the results obtained in this study.
Another limitation refers to the population studied, which may be representative of a Mediterranean, but not a broad European population due to the use of open footwear in the former because of a warmer climate. To address this limitation, it was ensured that data collection took place during the winter months when people wear only closed footwear, so as to avoid footwear use being a confounding variable. Furthermore, the participants have undergone a diabetes education program which specifically advised diabetes patients not to wear flip flops or go barefoot as part of their daily routine.
The authors are convinced that the sample population was correctly diagnosed as having no neuropathy since they were screened using standard tools, as recommended by common guidelines. However, a quantitative measurement of the level of neuropathy, e.g. by using the Neuropathy Disability Score or Michigan Neuropathy Screening Instrument, was not performed.
6. Conclusions
The results of this study may be important for screening purposes. They are also novel since this is first report on the occurrence of elevated peak plantar pressure in a commonly affected area of ulceration at the forefoot in participants living with diabetes with no obvious signs of deformity. These results confirm that diabetes itself may be a cause of increased peak plantar pressure and pressure time integral. Therefore, it is recommended that patients who have been living with this condition for more than 10 years should be regularly checked for increased peak plantar pressure even in the absence of foot deformity or other foot conditions. Today, advances in foot pressure mapping technology, which makes this examination procedure more accessible to practitioners, have made this possible.
Disclosures: The authors reported no conflict of interests.
References
- Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. In: Vascular health and risk management. 2007. p. 65-76. [DOD]
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005. 293(2):217-228. [DOD] [CrossRef]
- Tudhope L. The diabetic foot: recognition and principles of management. CME 2009. 27(7):312-315. [DOD]
- Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility (LJM) in elderly subjects with type II diabetes mellitus. Arch Gerontol Geriatr 2011. 53(2):135-140. [DOD] [CrossRef]
- Zimny S, Schatz H, Pfohl M. The Role of Limited Joint Mobility in Diabetic Patients with an At-Risk Foot. Diabetes Care 2004. 27(4):942-946. [DOD] [CrossRef]
- van Schie CH. A review of the biomechanics of the diabetic foot. Int J Low Extrem Wounds 2005. 4(3):160-170. [DOD] [CrossRef]
- Bus SA, Yang QX, Wang JH, Smith MB, Wunderlich R, Cavanagh PR. Intrinsic muscle atrophy and toe deformity in the diabetic neuropathic foot: a magnetic resonance imaging study. Diabetes Care 2002. 25(8):1444-1450. [DOD] [CrossRef]
- Sala D, Zorzano A. Differential control of muscle mass in type 1 and type 2 diabetes mellitus. Cell Mol Life Sci 2015. 72(20):3803-3817. [DOD] [CrossRef]
- Anjos DM, Gomes LP, Sampaio LM, Correa JC, Oliveira CS. Assessment of plantar pressure and balance in patients with diabetes. Arch Med Sci 2010. 6(1):43-48. [DOD] [CrossRef]
- Tuna H, Birtane M, Guldiken S, Soysal NA, Taspinar O, Sut N. The Effect of disease duration on foot plantar pressure values in patients with type 2 diabetes mellitus. Turkiye Fiz Tip ve Rehabil Derg 2014. 60(3):231-235. [DOD]
- Mueller MJ, Hastings M, Commean PK, Smith KE, Pilgram TK, Robertson D, Johnson J. Forefoot structural predictors of plantar pressures during walking in people with diabetes and peripheral neuropathy. J Biomech 2003. 36(7):1009-1017. [DOD] [CrossRef]
- Stess RM, Jensen SR, Mirmiran R. The role of dynamic plantar pressures in diabetic foot ulcers. Diabetes Care 1997. 20(5):855-858. [DOD] [CrossRef]
- Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care 1998. 21(10):1714-1719. [DOD] [CrossRef]
- Shaw JE, van Schie CH, Carrington AL, Abbott CA, Boulton AJ. An analysis of dynamic forces transmitted through the foot in diabetic neuropathy. Diabetes Care 1998. 21(11):1955-1959. [DOD] [CrossRef]
- Pitei DL, Lord M, Foster A, Wilson S, Watkins PJ, Edmonds ME. Plantar pressures are elevated in the neuroischemic and the neuropathic diabetic foot. Diabetes Care 1999. 22(12):1966-1970. [DOD] [CrossRef]
- Pataky Z, Assal JP, Conne P, Vuagnat H, Golay A. Plantar pressure distribution in type 2 diabetic patients without peripheral neuropathy and peripheral vascular disease. Diabet Med 2005. 22(6):762-767. [DOD] [CrossRef]
- Caselli A, Pham H, Giurini JM, Armstrong DG, Veves A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care 2002. 25(6):1066-1071. [DOD] [CrossRef]
- Bus SA, Lange A de. A comparison of the 1-step, 2-step, and 3-step protocols for obtaining barefoot plantar pressure data in the diabetic neuropathic foot. Clin Biomech 2005. 20(9):892-899. [DOD] [CrossRef]
- D'Ambrogi E, Giacomozzi C, Macellari V, Uccioli L. Abnormal foot function in diabetic patients: the altered onset of Windlass mechanism. Diabet Med 2005. 22(12):1713-1719. [DOD] [CrossRef]
- Formosa C, Gatt A, Chockalingam N. A critical evaluation of existing diabetic foot screening guidelines. Rev Diabet Stud 2016. 13(2-3):158-186. [DOD] [CrossRef]
- Gastwirth BW. Biomechanical examination of the foot and lower extremities. In: Clinical biomechanics of the lower extremities. Valmassy Rl (ed). 1st ed., St Louis, Mosby, 1996. [DOD]
- Redmond AC, Crosbie J, Ouvrier R. Development and validation of a novel rating system for scoring standing foot posture: The Foot Posture Index. Clin Biomech 2006. 21(1):89-98. [DOD] [CrossRef]
- Zammit GV, Menz HB, Munteanu SE. Reliability of the TekScan MatScan system for the measurement of plantar forces and pressures during barefoot level walking in healthy adults. J Foot Ankle Res 2010. 3(1):11. [DOD] [CrossRef]
- O'Sullivan K, Kennedy N, O'Neill E, Ni Mhainin U. The effect of low-dye taping on rearfoot motion and plantar pressure during the stance phase of gait. BMC Musculoskelet Disord 2008. 9(1):111. [DOD] [CrossRef]
- van der Leeden M, Dekker JH, Siemonsma PC, Lek-Westerhof SS, Steultjens MP. Reproducibility of plantar pressure measurements in patients with chronic arthritis: a comparison of one-step, two-step, and three-step protocols and an estimate of the number of measurements required. Foot Ankle Int 2004. 25(10):739-744. [DOD] [CrossRef]
- Fawzy OA, Arafa AI, El Wakeel MA, Abdul Kareem SH. Plantar pressure as a risk assessment tool for diabetic foot ulceration in Egyptian patients with diabetes. Clin Med Insights Endocrinol Diabetes 2014. 7:31-39. [DOD] [CrossRef]
- Gefen A. Plantar soft tissue loading under the medial metatarsals in the standing diabetic foot. Med Eng Phys 2003. 25(6):491-499. [DOD] [CrossRef]
- Pham HT, Exelbert L, Segal-Owens AC, Veves A. A prospective, randomized, controlled double-blind study of a moisturizer for xerosis of the feet in patients with diabetes. Ostomy Wound Manage 2002. 48(5):30-36. [DOD]
- Brash PD, Foster JE, Vennart W, Daw J, Tooke JE. Magnetic resonance imaging reveals micro-haemorrhage in the feet of diabetic patients with a history of ulceration. Diabet Med 1996. 13(11):973-978. [DOD] [CrossRef]
- Formosa C, Gatt A, Chockalingam N. The importance of clinical biomechanical assessment of foot deformity and joint mobility in people living with type-2 diabetes within a primary care setting. Prim Care Diabetes 2013. 7(1):45-50. [DOD] [CrossRef]
|