Original Data
| Rev Diabet Stud,
2007,
4(2):112-120 |
DOI 10.1900/RDS.2007.4.112 |
Increased Expression of Monocyte CD11b (Mac-1) in Overweight Recent-Onset Type 1 Diabetic Children
Vincenza Cifarelli1, Ingrid M. Libman2, Angela DeLuca3, Dorothy Becker2, Massimo Trucco1, Patrizia Luppi1
1Division of Immunogenetics, Department of Pediatrics, Children´s Hospital of Pittsburgh, University of Pittsburgh, School of Medicine, Pittsburgh, PA 15213, USA.
2Division of Endocrinology, Department of Pediatrics, Children´s Hospital of Pittsburgh, University of Pittsburgh, School of Medicine, Pittsburgh, PA 15213, USA.
3Department of Experimental Medicine, Section of Human Anatomy, University of Palermo, School of Medicine, Italy.
Address correspondence to: Patrizia Luppi, e-mail: luppip+@pitt.edu
Keywords: monocytes, adhesion molecules, type 1 diabetes, obesity, inflammation
Abstract
AIM: Compelling evidence implicates inflammation in the pathogenesis of type 1 diabetes mellitus (T1DM) and associated vascular complications. Obesity is also characterized by low-grade systemic inflammation. In this study, we characterized the inflammatory response in diabetes by analyzing the expression of a panel of activation markers on the surface of peripheral blood monocytes in recently-diagnosed T1DM patients. The potential effects of glycemic control and body mass index (BMI) on monocyte phenotype were also investigated. METHODS: Using flow cytometry, we analyzed the expression of CD11b, CD49d, CD54, CD62L and CD64 antigens on monocytes in a cohort of 51 T1DM patients (≤ 2 months after diagnosis). To test whether phenotype change in monocytes was associated with abnormal cellular function, we studied the adhesive capacity of monocytes to human umbilical vein endothelial cells (HUVEC). RESULTS: We found that circulating monocytes from T1DM patients tested at the clinical onset of the disease (i.e. within 1 week of diagnosis) had higher CD11b expression compared to patients analyzed 2 months after diagnosis (p = 0.02). The highest CD11b levels were detected in patients with HbA1c > 8% (p = 0.04 vs. patients with HbA1c < 8%). In T1DM children analyzed 2 months after diagnosis, we found that those who were overweight (BMI ≥ 85th percentile) had higher levels of monocyte activation than those who were not (BMI ≤ 85th percentile) (p = 0.03). CD11b and HbA1c were significantly correlated (correlation coefficient 0.329, p = 0.02). Finally, monocytes from T1DM patients showed higher adhesion to HUVEC compared to controls. CONCLUSIONS: Circulating immune cells from T1DM patients display many aspects of a proinflammatory state, as indicated by primed or activated monocytes. Obesity is an important factor in monocyte activation during diabetes.
Introduction
Compelling evidence demonstrates that components of the innate immune system, including natural killer cells (NK) and monocytes are involved in the autoimmune response characteristic of T1D both in humans and in the non-obese diabetic (NOD) mouse [1-4]. The primary role of monocytes in T1D has been demonstrated by showing that these cells are the first to accumulate in the pancreatic islets of prediabetic BB rats [5]. Subsequent T and B lymphocyte infiltration is dependent upon prior monocyte invasion of the islets [5]. These data suggest a role for monocytes in the early stages of T1D pathogenesis [6].
Monocytes are pivotal cells in inflammatory responses as they serve as the principal reservoir of pro-inflammatory cytokines and are the first cells to be engaged in nonspecific immune responses, such as those triggered by environmental factors. Recent studies reported evidence of increased monocytic activity, biomarkers of inflammation and oxidative stress in adult T1D patients well after the onset of diabetes [7, 8]. In these patients, monocytes also released higher levels of pro-inflammatory cytokines as compared to non-diabetic subjects, suggesting presence of an inflammatory response in T1D. Interestingly, elevated circulating levels of the pro-inflammatory cytokine interleukin (IL)-8 were found in children with recent-onset T1D (< 1 year) who were also overweight [9]. Other evidence of monocyte involvement in T1D includes studies showing aberrant constitutive and lipopolisaccharide (LPS)-stimulated expression of monocyte ciclooxygenase (COX)-2 expression in monocytes of T1D patients, a defect which may predispose to a chronic inflammatory response in T1D [4, 10]. The direct consequences of monocyte activation in T1D are unknown, but theoretically could involve release of pro-inflammatory cytokines, endothelial activation with increased monocyte adherence to the vascular endothelium, such as that of the pancreatic islets or kidney and retina capillaries.
During cellular activation, monocytes undergo phenotypic modifications with changes in expression of adhesion molecules on the cellular surface. These changes allow adhesion to the endothelial cells and movement of the monocytes through the endothelial layer toward inflammatory sites. The integrin Mac-1 (CD11b) is one of the most studied leukocyte adhesion molecules mediating tight binding to endothelial cells and migration through the vascular wall. While it is known that hyperglycemia, as seen in T1D, upregulates the expression of endothelial cell adhesion molecules [11-13], changes in adhesion molecule on the circulating monocytes have not been as well studied as those on the vascular endothelium. One report demonstrates increased expression of monocyte CD11b in adult T1D patients [14]. This increased CD11b expression was associated with higher monocyte adhesion to human aortic endothelial cells (HAEC) in vitro [14]. In contrast, other reports show either no increase [7, 15] or increased binding of monocytes to endothelial cells compared to healthy subjects [16].
The mechanism involved in the recruitment of monocytes in the peri-islet vasculature during diabetes is not fully understood. This is of particular importance in human subjects, because it is not possible to follow directly the different stages of the islet inflammatory process. Changes that affect both the vasculature and circulating monocytes in the early stages of T1DM may play a crucial role in promoting leukocyte adherence to the endothelium and ongoing infiltration of the islets. Another factor that could affect monocyte activation in T1DM patients is changes in body weight, as obesity has been associated with the presence of leukocyte abnormalities and inflammation [9, 17, 18]. In this study, we therefore investigated the potential role of glycemic control and body mass index (BMI) on monocyte expression of a panel of adhesion molecules in recently-diagnosed T1DM children (≤ 2 months after diagnosis).
Materials and Methods
Subjects
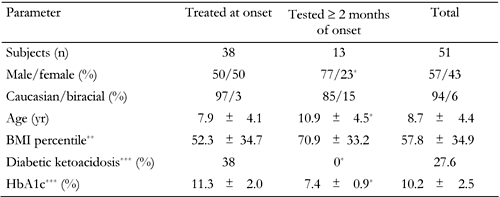
Fifty-one T1DM patients with recently diagnosed type 1 diabetes (T1DM) (i.e. within two months of the first insulin injection) (mean age 8.7 ± 4.4 SD, 22 F: 29 M) were randomly selected from those treated in the Diabetes Center at the Children's Hospital of Pittsburgh (CHP) between the years 2003-2004. Of those patients, thirty-eight were tested within one week of clinical diagnosis (defined as "new-onset"), while thirteen were tested at 2 months from clinical diagnosis. In the new-onset group, twelve patients had diabetic ketoacidosis (DKA), as defined by the presence of blood bicarbonate levels < 18 meq/l. The demographic and clinical characteristics of the population studied are shown in Table 1. Of the fifty-one diabetic patients, twenty-three tested positive for at least one autoantibody: GAD65, insulinoma-associated protein 2 (IA-2) and/or insulin autoantibodies (IAA) [19]. Each of the patients gave his informed consent to participate in the study, which was approved by the Children's Hospital Institutional Review Board.
Table
1.
Demographic and clinical characteristics of the T1DM population studied |
|
 |
 |
Legend:
Data are mean ± SD, numbers or percentages. BMI: body mass index. * p < 0.05 for comparisons between the two groups. ** Data available in 44 children (31 in group 1 and 13 in group 2). *** Data available in 47 children (34 in group 1 and 13 in group 2). |
|
Sample processing
Peripheral venous blood from T1DM patients was collected in sodium heparin tubes, kept at room temperature and processed within three hours for flow cytometry staining. Monocyte isolation was performed on Ficoll-isolated peripheral blood mononuclear cells (PBMC) and used for adhesion experiments as described below.
Flow cytometry
Sample labeling was performed as follows. Fifty microliters of whole blood from each subject were incubated with 20 μl of the appropriate dilutions of anti-human monoclonal antibodies (mAbs) and isotype matching mAbs for 15 minutes, in the dark, at room temperature. After lysing the erythrocytes with 2ml 1x PharM LyseTM (BD Pharmingen, San Diego, CA), samples were stored in 1% paraformaldehyde at 4°C until measured by flow cytometry between 5 minutes and 4 hours later [20].
The following anti-human mAbs were used: phycoerythrin (PE)-conjugated mAb to -CD62L (BD Pharmigen), -CD54 (BD Biosciences, San Diego, CA), and -CD49d (BD Pharmigen), Cy-chrome-conjugated mAb to -CD64, and -CD11b/Mac-1 (BD Pharmigen) and allophycocyanin-(APC)-conjugated mAb to -CD14 (BD Biosciences). Each mAb was used at saturating concentrations optimized by initial titrations for flow cytometry staining. The negative control panels were comprised of mixtures of isotype controls (BD PharMingen, BD Biosciences) diluted to an identical immunoglobulin concentration.
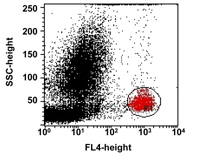
Flow cytometry and data analysis were undertaken as follows. Blood cells were analyzed using a Becton Dickinson (San Jose, CA) FACSCalibur machine calibrated with CaliBRITETM beads. At least 30,000 events were collected for each sample and the data were saved for later analysis on CELLQuest software (Becton Dickinson). Analysis of the expression of CD11b on the surface of circulating monocytes was performed by setting specific gates for the monocyte population based on dot plot graphs of side scatter (SSC) versus the positive cells for the monocyte-specific surface marker CD14 (Figure 1) [20]. For each population, a matched isotype control mAb was used to define the placement of the negative marker. Fluorescence was measured using a log10 scale. Since the mAb to the different adhesion molecules was used at saturating concentration, surface expression of this molecule could be quantitated as a measure of mean fluorescence intensity (MFI). MFI indicates the mean fluorescence value of cells positive for a marker, excluding outliers.
 |
 |
Figure 1. Gating parameters for flow cytometry analysis of adhesion molecule expression on CD14+ cells. For each sample tested, a gate (R1) was drawn for the monocyte population based on side scatter (SSC) characteristics versus the positive cells for the antibody specific to the monocyte surface marker CD14 (FL4). |
|
Statistical analysis
Comparisons of continuous variables were performed using the Mann-Whitney test. Spearman's rank correlation test was performed to examine associations between parameters tested. We considered p-values < 0.05 to be statistically significant. Computations were done using the SSPS statistical software package (SPSS, Chicago, IL).
Results
Monocytes from new-onset T1DM patients have an activated phenotype
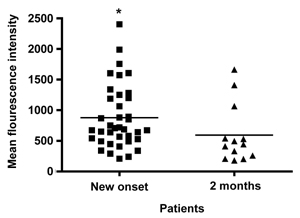
Analysis of the mean fluorescence intensity (MFI) of the monocyte marker CD11b in children with T1DM, showed a higher expression in those who were tested at the clinical onset of diabetes (n = 38, within 1 week of diagnosis) (878 ± 83 SEM, range 210-2403, median 698) compared to those tested at 2 months after diagnosis (n = 13) (595 ± 132 SEM, range 180-1663, median 442), (p = 0.02) (Figure 2 and Figure 3).
 |
 |
Figure 2. Expression of CD11b on monocytes in T1DM patients. A column-scatter-plot shows mean fluorescence intensity (MFI) of CD11b expression in circulating monocytes in recently-diagnosed T1DM patients. Analysis was performed by flow cytometry. Monocytes from T1DM patients at the onset of the disease (n = 38) show higher expression of the adhesion molecule CD11b than those from T1DM patients assayed at 2 months of diabetes (n = 13) (p = 0.02). Horizontal bars show mean CD11b values. * Represents significant differences in surface expression for CD11b between the two groups. |
|
 |
 |
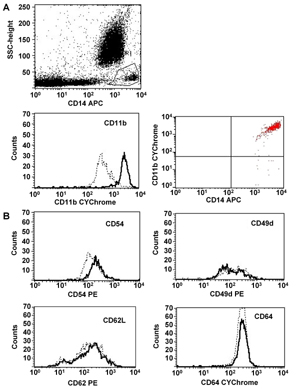
Figure 3. Representative flow cytometry analysis of CD11b expression in a T1DM patient. A shows a representative dot plot graph of CD11b expression in the circulating monocytes (CD14+) of T1DM patients at the onset of the disease. For each sample tested, dot plot graphs of SSC versus CD14+ cells were drawn and a tight gate was created, encompassing the "bright" elements for this specificity. Within the CD14 gate, CD14+ elements expressing the different adhesion molecules were analyzed by drawing separate dot plots. Percentage and MFI of positive cells was then defined by setting lower-limits for adhesion molecule positivity from each antibody combination using negative isotype controls. In A, a representative histogram of CD11b expression in T1DM patient at the onset of the disease and after 2 months from diagnosis is also shown. Expression of CD11b was higher in the T1DM patient at the onset of diabetes than after 2 months. B shows representative histograms of adhesion molecule expression in T1DM patients at the onset of the disease and 2 months after diagnosis. The x axis is fluorescence intensity of the stated adhesion molecule. Dotted line = T1DM patient 2 months after diagnosis, solid line = T1DM patient at the clinical onset. There were no significant differences in expression of CD54, CD49d, CD62L, and CD64 in monocytes between the two groups studied. |
|
Because diabetes ketoacidosis (DKA) has been described as an inflammatory condition characterized by elevated levels of C-reactive protein, within our cohort of newly-onset T1DM patients, we found that monocyte expression of CD11b in the children with DKA tended to be higher (1007 ± 188 SEM) compared to those without DKA (797 ± 82 SEM). However, this was not statistically significant (p = 0.67). When we compared those without DKA at onset (n = 21) with those without DKA at 2 months (n = 13), differences in the monocyte expression of CD11b was statistically significant between the two groups (797 ± 82 versus 595 ± 132 respectively, p = 0.03).
As expected, glycemic control measured by HbA1c was significantly higher in the onset group compared to the group tested at 2 months (11.3% ± 2 versus 7.4% ± 0.9, p = 0.0001). To test whether patients with higher HbA1c had also higher monocyte activation, we compared the expression of monocyte CD11b in T1DM patients according to HbA1c levels. We found that T1DM patients with higher HbA1c levels (HbA1c > 8%) (n = 35) had significantly higher expression of CD11b (896 ± 75 SEM) as compared to those with lower HbA1c levels (HbA1c ≤ 8%) (563 ± 103 SEM) (n = 12) (p = 0.04). In addition, CD11b and HbA1c were significantly correlated (correlation coefficient 0.329, p = 0.02).
Analysis of the expression of the surface antigens CD49d, CD54, CD62L and CD64 on circulating monocytes in the different subgroups of patients did not reveal any significant differences. A representative example of the expression of the stated markers in T1DM patients at the onset of the disease and 2 months after diagnosis is shown in Figure 3.
Overweight children with T1DM have high monocyte expression of CD11b
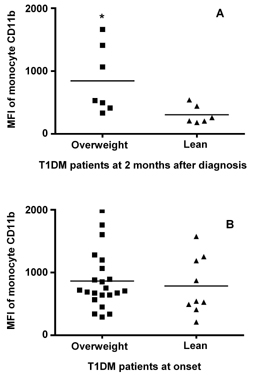
There is evidence that obesity is associated with a systemic inflammatory process involving both leukocytes and body fat [17, 18]. We therefore analyzed changes in monocyte CD11b expression in T1DM according to BMI. We found that among T1DM children, who were tested 2 months after diagnosis (n = 13), expression of CD11b was significantly higher in overweight children (BMI ≥ 85th percentile) (n = 7) (844 ± 201 SEM, median 531, range 332-1663) as compared to children who were not overweight (BMI ≤ 85th percentile) (n = 6) (305 ± 61 SEM, median 233, range 180-540) (p = 0.03) (Figure 4A). These two groups were no different in terms of their glycemic control as measured by HbA1c (7.1% ± 0.3 in the overweight group versus 7.6% ± 4.5 in the non-overweight group, p = 0.33) and none of them were in DKA at the time of the evaluation.
The same analysis performed in patients at the onset of the disease (n = 31) did not show significant differences in monocyte CD11b between overweight (BMI ≥ 85th percentile) (n = 9) (864 ± 97 SEM, median 713, range 291-1989) and lean (BMI ≤ 85th percentile) (784 ± 153 SEM, median 543, range 210-1574) (n = 22) patients p = 0.66 (Figure 4B). These two groups were no different in terms of their control as measured by HbA1c (10.4% ± 0.5 in the overweight group versus 11.6% ± 0.4 in the non-overweight group, p = 0.33) or the presence of DKA (50 versus 31%, p = 0.65). CD11b and BMI percentile did not correlate in a statistically significant fashion (correlation coefficient 0.027, p = 0.86).
 |
 |
Figure 4. Monocyte CD11b expression among T1DM patients is higher in those who are overweight. In A, a column-scatter-plot shows mean fluorescence intensity (MFI) for the molecule CD11b in monocytes according to body mass index (BMI) in thirteen patients tested 2 months after diagnosis (lean n = 6, overweight n = 7). In B, the same analysis is performed in thirty-one T1DM patients at the onset of the disease (lean n = 22, overweight n = 9). Horizontal bars show mean CD11b values. * Represents significant differences in cell surface expression for CD11b between T1DM patients who are overweight (BMI ≥ 85th percentile) and lean (BMI ≤ 85th percentile). |
|
Conclusions
Little is known about monocyte phenotype and function in children with recently diagnosed T1DM. In this study, we analyzed the expression of a panel of adhesion molecules on circulating monocytes in T1DM children within 2 months of clinical diagnosis and evaluated the potential role of glycemic control and BMI on cell phenotype. We found that, among the markers analyzed, only the expression of CD11b was significantly higher in the cohort of new-onset diabetic patients (i.e. within 1 week of diagnosis) as compared to subjects tested after 2 months after diagnosis. CD11b is a polypeptide α-chain linked to the β2-subunit of CD18 that constitutes the CD11/CD18 β2-integrin family. Resting monocytes constitutively express integrins, which are important signal transducers for virtually all monocyte functions, by mediating cell adhesion, chemotaxis, migration, phagocytosis, and oxidant production. After monocyte activation, new copies of CD11b/CD18 are rapidly translocated to the cell surface from the intracellular granules [21]. Our finding of increased CD11b expression on monocytes at the onset of T1DM suggests the presence of immune activation at such an early stage of the disease. This result also supports an earlier finding of increased monocyte CD11b expression in T1DM patients [14].
Within the cohort of new-onset T1DM patients, children who presented with DKA had the highest levels of monocytes CD11b detected. Although these data did not reach statistical significance, most likely because of small numbers, it supports a previous report showing presence of an inflammatory response in T1DM children with DKA [22]. However, other factors besides DKA appear to be involved in triggering monocyte activation at the onset of diabetes. In fact, significantly higher monocyte CD11b expression was detected in new-onset T1DM children without DKA versus those without DKA at 2 months. These results seem to suggest that the onset of diabetes is per se an inflammatory condition. Further studies on a larger number of patients at the onset of T1DM are required to define better the inflammatory state present in DKA.
We found that diabetic children tested 2 months after diagnosis, who were overweight (BMI ≥ 85th percentile), displayed higher CD11b values when compared to lean diabetic children. This finding supports the association between obesity and inflammation [17, 18]. Recently, it has been shown that obesity is characterized by abnormalities in peripheral leukocyte counts [17], increased circulating levels of the pro-inflammatory cytokine interleukin (IL)-8 [9] and by an accumulation of immune cells, especially macrophages, in the adipose tissue [18]. The fact that we could not detect the same changes in monocyte CD11b between overweight and lean diabetic children within the cohort of patients tested at the onset of the disease is probably due to the confounding effect of the inflammatory response associated with the onset of the disease.
The factor(s) triggering upregulation of CD11b in the monocytes in diabetes and its biological significance are not known. One hypothesis would be that the increased expression of CD11b in monocytes in diabetes is simply a marker of an inflammatory response, which becomes more pronounced in overweight children and in children who present with DKA. Alternatively, CD11b upregulation could reflect an increased monocyte activation in response to the poor glycemic control and insulin deficiency which is likely to be present at the onset of diabetes, a hypothesis supported by the fact that HbA1c levels were significantly higher at the onset than at 2 months and by the fact that there was a significant correlation between the two variables. In addition, diabetic children with higher HbA1c (HbA1c > 8%) also had higher CD11b. A third explanation envisions that intercurrent infections may have precipitated the diagnosis (because of increased insulin requirements) and be responsible for immune activation. At this point, we cannot decide to what extent the inflammatory state is a pathogenic mechanism contributing to the development of T1DM or is the response to a metabolic derangement. In the first case, the inflammatory state should precede overt diabetes and, in this context, the study of pre-diabetic subjects (i.e. autoantibody-positive individuals) should be characterized in respect to the inflammatory state.
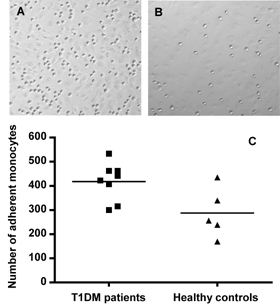
Our findings of increased CD11b in the monocytes of T1DM patients at the clinical onset of diabetes would strengthen the idea that these cells have the potential to adhere to the endothelium and exit the circulation at such an early stage of the disease. In fact, current investigations in our laboratory suggest that monocytes isolated from the peripheral blood of recently-diagnosed T1DM patients (who were free from any vascular complications) have increased adherence to human umbilical vein endothelial cells (HUVEC) in vitro (Figure 5). These unpublished findings are preliminary, but they support the hypothesis that monocytes from recent-onset T1DM patients have increased adhesive capacity. Protracted inflammation with increased expression of the same adhesion molecules could be the basis for late diabetes-associated atherosclerotic vascular complications.
 |
 |
Figure 5. Monocytes from T1DM patients have increased adherence to human umbilical vein endothelial cell (HUVEC). Freshly isolated monocytes (1x106 cells/ml) from new-onset T1DM patients (n = 8) and from healthy controls (n = 5) were added to confluent HUVEC in 48 well plates and incubated for 1h at room temperature on a rocking plate. Each experiment was performed in triplicate. After washing away non-adherent cells, adherent monocytes were fixed with 1.25% gluteraldehyde at 4ºC overnight. Next day, monocytes were stained with 0.4% trypan blue and cells were counted. To determine the number of monocyte adherent to HUVEC in each donor, five random 40x fields per well were counted and averaged. 40x representative photographs of monocytes adherent to HUVEC in T1DM patient (A) and non-diabetic control subjects (B) are shown. C gives a column-scatter-dot-plot showing the number of monocytes adherent to HUVEC in 5 random 40x fields per well. Horizontal bars show the mean number of monocytes. The number of monocytes adherent to the wells in T1DM patients was significantly higher than in normal controls (p = 0.04). |
|
In conclusion, the clinical onset of T1DM is associated with changes in activation-related markers in the circulating monocytes as part of a generalized inflammatory response. We have identified the leukocyte integrin CD11b/CD18 as a crucial molecule upregulated in circulating monocytes, especially in overweight children. Enhanced monocyte CD11b would support the notion that monocytes in recently diagnosed T1DM patients have the potential to adhere to the endothelium, most likely in the vasculature of the pancreas, and to accumulate in the islets.
Acknowledgments:
We thank the nurses of the Diabetes Clinic and of the Endocrinology Division of the Diabetes Center at the Children’s Hospital of Pittsburgh for making blood samples from Type 1 diabetic patients promptly available to us. We also thank Dr. Sati Mazumdar, Department of Biostatistics, Graduate School of Public Health, for discussion on the statistical analysis. This study was supported by grant DK 024021-24 from the National Institute of Health and NIH 5K12 DK063704 and the Cochrane Weber Endowed Fund (I. Libman).
References
- Goodier MR, Nawroly N, Beyan H, Hawa M, Leslie RD, Londei M. Identical twins discordant for type 1 diabetes show a different pattern of in vitro CD56+ cell activation. Diabetes Metab Res Rev 2006. 22(5):367-375. [DOD] [CrossRef]
- Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, et al. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature 1998. 391:177-181. [DOD] [CrossRef]
- Adler T, Akiyama H, Herder C, Kolb H, Burkart V. Heat shock protein 60 elicits abnormal response in macrophages of diabetes-prone non-obese diabetic mice. Biochem Biophys Res Commun 2002. 294:592-596. [DOD] [CrossRef]
- Beyan H, Goodier MR, Nawroly NS, Hawa MI, Bustin SA, Ogunkolade WB, Londei M, Yousaf N, Leslie RD. Altered monocyte cyclooxygenase response to lipopolysaccharide in type 1 diabetes. Diabetes 2006. 55:3439-3445. [DOD] [CrossRef]
- Hanenberg H, Kolb-Bachofen V, Kantwerk-Funke G, Kolb H. Macrophage infiltration precedes and is a prerequisite for lymphocytic insulitis in pancreatic islets of pre-diabetic BB rats. Diabetologia 1989. 32:126-134. [DOD] [CrossRef]
- Kolb-Bachofen V, Kolb H. A role for macrophages in the pathogenesis of type 1 diabetes. Autoimmunity 1989. 2:145-154. [DOD]
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 2006. 55:774-779. [DOD] [CrossRef]
- Plesner A, Greenbaum CJ, Gaur LK, Ernst RK, Lernmark A. Macrophages from high-risk HLA-DQB1*0201/*0302 type 1 diabetes mellitus patients are hypersensitive to lipopolysaccharide stimulation. Scand J Immunol 2002. 56:522-529. [DOD] [CrossRef]
- Erbagci AB, Tarakcioglu M, Coskun Y, Sivasli E, Namiduru ES. Mediators of inflammation in children with type 1 diabetes mellitus: cytokines in type 1 diabetic children. Clinical Biochem 2001. 34:645-650. [DOD] [CrossRef]
- Litherland SA, Xie XT, Hutson AD, Wasserfall C, Whittaker DS, She JX, Hofig A, Dennis MA, Fuller K, Schatz D, et al. Aberrant prostaglandin synthase 2 expression defines an antigen-presenting cell defect for insulin-dependent diabetes mellitus. J Clin Invest 1999. 104:515-523. [DOD]
- Kado S, Wakatsuki T, Yamamoto M, Nagata N. Expression of intercellular adhesion molecule-1 is induced by high glucose concentrations in human aortic endothelial cells. Life Sci 2001. 68:727-737. [DOD] [CrossRef]
- Kim JA, Berliner JA, Natarajan RD, Nadler JL. Evidence that glucose increases monocyte binding to human aortic endothelial cells. Diabetes 1994. 43:1103-1107. [DOD] [CrossRef]
- Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kappaB-dependent fashion. J Clin Invest 1998. 101:1905-1915. [DOD]
- Kunt T, Forst T, Fruh B, Flohr T, Schneider S, Harzer O, Pfutzner A, Engelbach M, Lobig M, Beyer J. Binding of monocytes from normolipidemic hyperglycemic patients with type 1 diabetes to endothelial cells is increased in vitro. Exp Clin Endocrinol Diabetes 1999. 107:252-256. [DOD]
- Hoogerbrugge N, Verkerk A, Jacobs ML, Postema PT, Jongkind HF. Hypertriglyceridemia enhances Mo binding to Ec in NIDDM. Diabetes Care 1996. 19:1122-1126. [DOD] [CrossRef]
- Setiadi H, Wautier JL, Courillon-Mallet A, Passa P, Caen J. Increased adhesion to fibronectin and Mo-1 expression by diabetic monocytes. J Immunol 1987. 138:3230-3234. [DOD]
- Zaldivar F, McMurray RG, Nemet D, Galassetti P, Mills PJ, Cooper DM. Body fat and circulating leukocytes in children. Int J Obes 2006. 30:906-911. [DOD] [CrossRef]
- Sbarbati A, Osculati F, Silvagni D, Benati D, Galie M, Camoglio FS, Rigotti G, Maffeis C. Obesity and inflammation: evidence for an elementary lesion. Pediatrics 2006. 117:220-223. [DOD] [CrossRef]
- Pietropaolo M, Becker DJ, LaPorte RE, Dorman JS, Riboni S, Rudert WA, Mazumdar S, Trucco M. Progression to insulin-requiring diabetes in seronegative prediabetic subjects: the role of two HLA-DQ high-risk haplotypes. Diabetologia 2002. 45:66-76. [DOD] [CrossRef]
- Luppi P, Haluszczak C, Trucco M, DeLoia J. Normal pregnancy is associated with peripheral leukocyte activation. AJRI 2002. 47:72-81. [DOD]
- Miller LJ, Bainton DF, Borregaard N, Springer TA. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest 1987. 80:535-544. [DOD]
- Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crisis. Diabetes 2004. 53:2079-2086. [DOD] [CrossRef]
This article has been cited by other articles:

|
C-peptide and long-term complications of diabetes
Luppi P, Cifarelli V, Wahren J
Pediatr Diabetes 2011. Epub ahead of print
|
|

|
CD40 expression and its association with low-grade inflammation in a Greek population of type 1 diabetic juveniles: evidence for differences in CD40 mRNA isoforms expressed by peripheral blood mononuclear cells
Chatzigeorgiou AE, Lembessis PE, Mylona-Karagianni CF, Tsouvalas EA, Diamanti-Kandarakis E, Kamper EF
Exp Clin Endocrinol Diabetes 2010. 118(1):38-46
|
|

|
Anti-inflammatory properties of C-Peptide
Haidet J, Cifarelli V, Trucco M, Luppi P
Rev Diabet Stud 2009. 6(3):168-179
|
|

|
Cardiovascular complications of diabetes mellitus: The Tissue Factor perspective
Bogdanov VY, Osterud B
Thromb Res 2009. Jul 30, epub ahead of print
|
|

|
Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-kappaB pathway
Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M
Diabetologia 2008. 51(8):1534-1543
|
|

|
Human proinsulin C-peptide reduces high glucose-induced proliferation and NF-kappaB activation in vascular smooth muscle cells
Cifarelli V, Luppi P, Tse HM, He J, Piganelli J, Trucco M
Atherosclerosis 2008. 201(2):248-257
|
|
|