Original Data
| Rev Diabet Stud,
2007,
4(4):242-248 |
DOI 10.1900/RDS.2007.4.242 |
The Incidence of Microalbuminuria and its Associated Risk Factors in Type 2 Diabetic Patients in Isfahan, Iran
Massoud Amini, Hassan Safaei, Ashraf Aminorroaya
Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Address correspondence to: Ashraf Aminorroaya, e-mail: aminorroaya@med.mui.ac.ir
Manuscript submitted January 1, 2008; resubmitted February 17, 2008; accepted February 19, 2008.
Keywords: type 2 diabetes, microalbuminuria, diabetic nephropathy, incidence, risk factor
Abstract
AIM: The study was carried out to determine the five-year incidence of microalbuminuria and to assess its associated risk factors for type 2 diabetic patients in Isfahan, Iran. METHODS: 505 type 2 diabetic patients (22% male, 78% female) with normal urinary albumin levels, being treated at Isfahan Endocrine and Metabolism Research Center, were consecutively selected. After the initial selection in 1999, the patients were followed for five years. Mean and standard deviation (SD) of age and duration of diabetes was 57.4 (9.5) and 10.2 (4.7) years, respectively. BMI, blood pressure, fasting plasma glucose, HbA1c, serum lipids and serum creatinine were measured and re-examined every three months. 24-h urinary albumin excretion was measured and reviewed annually. Microalbuminuria was diagnosed when at least two measurements indicated the excretion of more than 30 mg albumin in 24-h urinary samples. RESULTS: During 5-year follow up, 176 patients developed microalbuminuria, giving an incidence rate of 82.3/1000 person/year (95% CI: 78.3-86.2). Males had a higher incidence than females (104.4 vs. 66.2/1000 person/year, p < 0.001). Duration of diabetes, abnormal levels of HbA1c, hypertension and high serum creatinine were significantly associated with microalbuminuria. There was no difference in mean of age, BMI, and lipid levels between patients with and without microalbuminuria. Multivariate analysis was used to show that duration of diabetes, HbA1c, hypertension and retinopathy were the independent variables related to microalbuminuria. CONCLUSIONS: The incidence of microalbuminuria in the study population was higher than in other populations. The higher incidence and the considerable gender difference in this population may be attributed to inferior glycemic control and lack in screening for risk factors, but this needs to be explored in further studies.
Introduction
Diabetic nephropathy is a common cause of renal failure. In some western countries, the incidence of diabetic nephropathy has increased by as much as 150% in the past decade [1-3]. The current global epidemic of diabetes means that the number of patients with renal failure due to diabetes will also rise dramatically. Forty percent of patients undergoing dialysis in 1998 had diabetic nephropathy, and of these patients 22% had a higher mortality rate in the first year following the start of dialysis compared with diabetic patients without renal involvement [4, 5]. The well known consequences are related medical problems and decreased quality of life for many patients, and a heavy financial burden on health care systems. Therefore, early detection of renal impairment and consequent treatment with preventive strategies is very important.
Microalbuminuria is often the first sign of renal involvement in diabetic patients which is, at this stage, classified as incipient nephropathy. On average, 20-40% of diabetic patients develop microalbuminuria 10 years after the onset of diabetes [6]. Microalbuminuria is a risk factor for overt nephropathy. Monitoring for this condition is important because the treatment of microalbuminuria can prevent or postpone overt nephropathy [7, 8]. The present study was carried out to investigate the incidence of microalbuminuria and to assess its determinants and association with other potential risk factors in type 2 diabetic patients in Isfahan, a major city located in Iran.
Patients and methods
558 patients with type 2 diabetes being treated at Isfahan Endocrine and Metabolism Research Center (IEMRC) took part in this prospective cohort study which started in 1999. The criterion for selection was that had a normal 24-h urinary albumin excretion at baseline, determined according to WHO-criteria (<30 mg albumin in 24 hours). The study was approved by the Research Ethics Committee of IEMRC and Isfahan University of Medical Sciences.
After written informed consent by all study participants, baseline data regarding demography, medical history and smoking behavior were collected. Physical examination consisted of the measurement of weight, height and blood pressure. Laboratory investigations included the measurement of fasting plasma glucose, HbA1c, serum lipoproteins and creatinine. As mentioned, baseline 24-h urine collection was performed with micro- or macroalbuminuria being exclusion criteria.
Subsequently, patients were re-examined at 3-month intervals to repeat clinical examinations and measurement of fasting plasma glucose, HbA1c, lipids and creatinine. 24-hour urinary albumin was measured once each year during the 5-year follow-up period. Weight and height were measured barefoot in light clothing and BMI was calculated as body weight (kg) divided by the square of height (m). A BMI less than 25 kg/m2 was considered as normal, BMI between 25 kg/m2 and 30.0 kg/m2 as overweight and greater than 30 kg/m2 as obesity [9]. Blood pressure was measured twice on the right arm in sitting position after 15 min of rest using a standard mercury sphygmomanometer. The mean of two readings, five minutes apart, was taken as the overall result. Hypertension was defined according to the Seventh Report of the Joint National Committee (7th JNC) with systolic blood pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg regarding as hypertension [10]. Patients already on treatment with antihypertensive medications were also considered to be hypertensive.
Blood samples were taken after 10 h of fasting to measure plasma glucose levels. Plasma glucose was measured using the glucose oxidase method. HbA1c was measured with a DSS machine using the ion exchange chromatography method. Total cholesterol, triglyceride and high-density lipoprotein (HDL) cholesterol were measured using Pars Azmoon and Chem Enzyme kits. Low-density lipoprotein (LDL) cholesterol was calculated with Friedenwald's formula (provided that triglyceride level was less than 400 mg/dl) [11]. LDL values were left out of the analysis in cases where there was an elevated triglyceride level. Adult Treatment Panel III (ATPIII) criteria [12] were used to classify plasma lipid levels. Total cholesterol, triglyceride and LDL levels exceeding 200, 150, and 100 mg/dl respectively, and HDL levels below 45 mg/dl were considered as abnormal.
In order to measure urinary albumin levels accurately, patients were trained how to collect urine samples. 24-h urinary creatinine was measured to ensure that correct volumes of urine had been collected. When no evidence of infection and/or hematuria was found in urinalysis, urine samples with specific gravity >1015 were examined for microalbuminuria. Urinary albumin was measured with an Autoanalyzer (Analyzer Medical Systems, Italy) using Randox kits (urinary albumin measurement with the immunoturbidimetry method, UK). A second 24-h urinary sample was obtained and examined for microalbuminuria if the first measurement exceeded 30 mg of albumin. The diagnosis of microalbuminuria was confirmed when more than 30 mg/dl albumin was found in the second sample.
24-h urinary albumin concentrations of <30 mg were considered as normal (normoalbuminuria), 30-300 mg as microalbuminuria and >300 mg as macroalbuminuria (overt proteinuria) [13]. Smoking was defined according to the WHO criteria. In this study, smokers were defined as individuals who regularly smoked at least one cigarette per day (daily smokers) [14].
Statistical analysis
Data are presented as mean and standard deviation (SD) with a normal distribution, and as median and 95th percentile with a skewed distribution. Continuous variables were compared using appropriate non-parametric tests. Quantitative variables were compared using Student's t-test. Univariate and multivaraiate logistic regression analysis was used to determine the risk factors of microalbuminuria. Data were analyzed with SPSS and p-values less than 0.05 were considered as statistically significant.
Results
A total of 111 men (22%) and 394 women (78%) completed the study. Fifty-three patients (9.5%) were excluded because they did not return for regular visits during follow-up. Mean age was 57.5 (SD 9.5) and mean duration of diabetes 10.2 (SD 4.5) years. Mean age was higher in men than women (59.4 ± 10.3 vs. 56.5 ± 8.9 years, p < 0.05). However, the duration of disease was comparable in men and women.
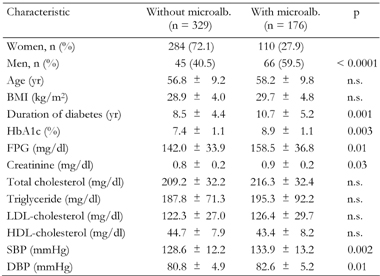
Table 1 shows the comparison between follow-up variables in patients with and without microalbuminuria. Mean duration of disease, fasting plasma glucose, HbA1c, blood pressure and plasma creatinine were all significantly higher in patients with microalbuminuria compared with patients with normal urinary albumin excretion. The prevalence of hypercholesterolemia and hypertriglyceridemia was 68.7% and 65.2%, respectively. LDL cholesterol was higher than 100 mg/dl in 79.8% of patients. However, none of the lipid parameters were associated with an increased risk of microalbuminuria.
Table
1.
Quantitative characteristics in type 2 diabetic patients with and without microalbuminuria during follow-up |
|
 |
 |
Legend:
Data are mean ± SD. FPG: fasting plasma glucose. SBP: systolic blood pressure. DBP: diastolic blood pressure. |
|
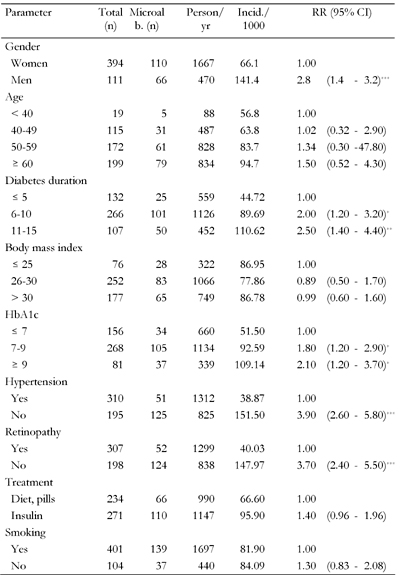
The 5-year incidence of microalbuminuria was 82.3/1000 person/year (95% CI: 69-105). The incidence of microalbuminuria in men was significantly higher than in women (141.4/1000 in men vs. 66.1/1000 in women, p < 0.001, Table 2).
Table
2.
Incidence and relative risk of microalbuminuria according to risk factor |
|
 |
 |
Legend:
* p < 0.05, ** p < 0.01, *** p < 0.001. |
|
Table 2 shows the incidence of microalbuminuria according to the risk factors identified by univariate regression analysis. Cut-off levels were selected on the basis of usual limits and not necessarily based on the results of the univariate analysis. Beside the obvious gender difference, the analysis showed the expected trend towards higher incidence of microalbuminuria with longer clinical duration of diabetes. An HbA1c of >7.0% was associated with an 1.8 times higher relative risk of developing microalbuminuria during the first five years. Similary, presence of hypertension means a 3.9-times higher risk. Furthermore, retinopathy (defined as existence of retinal lesions examined by retinal photography) and treatment with insulin were also significantly associated with an increased risk of progression to microalbuminuria.
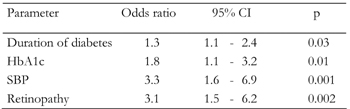
Since a number of the factors are interrelated, multivariate analysis is necessary to find out independent associations. Data from multivariate analysis with adjustment for other variables are presented in Table 3. The analysis showed that longer duration of diabetes, hypertension (systolic blood pressure of >140 mmHg and diastolic blood pressure of >90 mmHg), higher HbA1c and the presence of retinopathy were independently associated with a higher risk for developing microalbuminuria.
Table
3.
Independent variables determining microalbuminuria in type 2 diabetic patients |
|
 |
 |
Legend:
SBP: systolic blood pressure. |
|
Discussion
The findings of different studies are not readily comparable due to differences in diagnostic methods, age distribution, duration of diabetes and patient selection methods. In order to describe an adequate global picture of the development of microalbuminuria and diabetic nephropathy, data from different continents and patient groups need to be examined. The present study describes the incidence of new-onset microalbuminuria in type 2 diabetic patients in Isfahan, Iran, a sparsely examined region so far. In contrast to case-control-studies, which define differences in characteristics between patients with and without established microalbuminuria, this study examines factors associated with the development of this complication during the first five years after normoalbuminuria in the Iranian population. The present study showed that the incidence of albuminuria in the Iranian study population is higher than in other populations and that there is a significant gender difference.
A few other studies form different countries have also reported the incidence of new-onset microalbuminuria in type 2 diabetes. A prospective cohort study in Korea involving 188 type 2 diabetic patients without albuminuria reported an incidence rate of 52 per 1000 population after 5.5 years of follow-up [15]. An Indian study reported the 5-year incidence of microalbuminuria at 46.9 per 1000 population [16]. Nelson and colleagues reported the accumulative incidence of microalbuminuria in the Pima race at 37% after 4.7 years of follow-up [17] In the present study, we observed a rate of 34.8% of the patients with follow-up developed microalbuminuria. This rate equals 82.3/1000 patient years exceeding the incidence from other studies. The reason for this considerable difference in the incidence rates is not fully understood. It may be partly related to suboptimal risk factor detection and treatment. Due to the association of diabetes with the development of microalbuminuria we suppose that inferior glycemic control may play a role, too. However, we found no study in the literature focusing on the incidence of microalbuminuria and glycemic control in diabetic patients in Iran. This would be an interesting aspect for future research.
The present study confirmed known predictive factors which were previously found in other studies. These factors are hypertension, duration of disease, higher HbA1c, retinopathy and being male. However, if found to be consistent, both the higher incidence of microalbuminuria and especially the gender difference raise questions about the medical and/or non-medical pathophysiology of this specific complication in this population. Non-medical factors pertain to the accessibility and use of health care facilities, compliance with therapy and lifestyle factors. Medical factors include the detection and treatment of the described risk factors. However, association with the development of microalbuminuria does not always indicate causality. The association with retinopathy, for example, may be another reflection of microvascular disease. Causal factors are predestined for intervention if a positive effect on a relevant end point has been shown in randomized trials. However, some potential causal factors can not be redressed like duration of disease.
Some studies investigated the effect of age on the incidence of albuminuria [18, 19]. Bruno et al. reported that increasing age was independently associated with microalbumiuria in an Italian population, even with adjustment for duration of disease [18]. In contrast, an association between age and albuminuria could not be confirmed with our study design in the Iranian population, even in univariate analysis. An association between age and albumin excretion rate was also not found in an Afro-American population [19].
The incidence of albuminuria in our study increased with duration of diabetes, which was found to be one of the determinants of albuminuria after adjustment for other variables. This result corresponds with other studies [20-22]. As duration of diabetes increases with age, actually both variables should be related to nephropathy. However, duration of disease is difficult to record in type 2 diabetes since it is well-known that hyperglycemia usually starts 4-7 years before the clinical diagnosis of diabetes [23]. We found mean fasting plasma glucose in patients with abnormal urinary albumin to be higher than in those with normal urinary albumin. HbA1c is a more accurate indicator of glycemic control and has been shown to be an independent risk factor.
The incidence of microalbuminuria in men was found to be significantly higher than in women, which is in agreement with a Danish study [24]. The reason for this difference is not clear. In line with the current study, several research groups have highlighted hyperglycemia and hypertension as major risk factors for microalbuminuria and nephropathy in diabetic patients [25-33]. Hypertension was also one of the major determinants of microalbuminuria in the present study. Univariate analysis revealed significantly higher levels of systolic and diastolic blood pressure in patients with microalbuminuria compared with patients without microalbuminuria. Adequate blood pressure control decreases the incidence of microalbuminuria, and retards the progression of disease towards overt nephropathy [34]. This suggests that emphasis should be placed on the use of inhibitors of the renin-angiotensin-aldosteron system [35-37].
In the present study, the incidence of albuminuria in patients with retinopathy was greater than in those without. Recent studies have demonstrated diabetic nephropathy to increase the ocular complications of the disease, including retinopathy [38-40].
Conclusions
In conclusion, the 5-year incidence of microalbuminuria in our study population was significantly higher than in other countries. It would be useful to confirm this result and compare its causes with results from other countries. Also, the analysis of contributing medical and non-medical risk factors should be extended. Nevertheless, it is clear that the incidence of microalbuminuria, and late-stage macroalbumiuria requiring renal replacement therapy, can be modified by appropriate and timely actions, with great savings in human and economic costs. The control of modifiable risk factors, especially hyperglycemia and hypertension, as well as timely detection and treatment of incipient nephropathy can decrease the incidence of albuminuria in diabetic patients.
References
- Vanrenterghem Y, Jones EH. Report on management of renale failure in Europe, XXVI, 1995. Report based on the Centre Questionnaire, 1995. The ERA-EDTA Registry. Nephrol Dial Transplant 1996. 11(Suppl 7):28-32. [DOD]
- Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999. 34:795-808. [DOD]
- Raine AE. Epidemiology, development and treatment of end-stage renal failure in type 2 (non-insulin-dependent) diabetic patients in Europe. Diabetologia 1993. 36:1099-1104. [DOD] [CrossRef]
- Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med 2002. 346:1145-1151. [DOD] [CrossRef]
- Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med 1996. 335(22):1636-1642. [DOD] [CrossRef]
- Remuzzi G, Schiepatti A, Ruggementi P. Nephropathy in patients with type 2 diabetes. New Engl J Med 2002. 342:1145-1151. [DOD] [CrossRef]
- Parving HH, Chaturvedi N, Viberti G, Mogensen CE. Does microalbuminuria predict diabetic nephropathy? Diabetes Care 2002. 25:406-407. [DOD]
- Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 1984. 310:356-360. [DOD]
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of consultation obesity. Geneva, 1998. [DOD]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003. 42:1206-1252. [DOD] [CrossRef]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972. 18:499-502. [DOD]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001. 285(19):2486-2497. [DOD] [CrossRef]
- Haffner SM, American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care 2003. 26(Suppl 1):S83-S86. [DOD]
- World Health Organization. Guidelines for controlling and monitoring the tobacco epidemic. Geneva, 1998. [DOD]
- Park JY, Kim HK, Chung YE, Kim SW, Hong SK, Lee KU. Incidence and determinants of microalbuminuria in Koreans with type 2 diabetes. Diabetes Care 1998. 21:530-534. [DOD] [CrossRef]
- John L, Rao PS, Kanagasabapathy AS. Rate of progression of albuminuria in type II diabetes. Five-year prospective study from south India. Diabetes Care 1994. 17(8):888-890. [DOD] [CrossRef]
- Nelson RG, Knowler WC, Pettitt DJ, Hanson RL, Bennett PH. Incidence and determinants of elevated urinary albumin excretion in Pima Indians with NIDDM. Diabetes Care 1995. 18:182-187. [DOD] [CrossRef]
- Bruno G, Cavallo-Perin P, Bargero G, Borra M, Calvi V, D'Errico N, Deambrogio P, Pagano G. Prevalence and risk factors for micro- and macroalbuminuria in an Italian population-based cohort of NIDDM subjects. Diabetes Care 1996. 19:43-47. [DOD] [CrossRef]
- Dasmahapatra A, Bale A, Raghuwanshi MP, Reddi A, Byrne W, Suarez S, Nash F, Varagiannis E, Skurnick JH. Incipient and overt diabetic nephropathy in African Americans with NIDDM. Diabetes Care 1994. 17(4):297-304. [DOD] [CrossRef]
- Oue T, Namba M, Nakajima H, Ono A, Horikawa Y, Yamamoto K, Hamaguchi T, Fujino-Kurihara H, Yamasaki T, Tomita K, Miyagawa J, Hanafusa T, Matsuzawa Y. Risk factors for the progression of microalbuminuria in Japanese type 2 diabetic patients--a 10 year follow-up study. Diabetes Res Clin Pract 1999. 46:47-55. [DOD] [CrossRef]
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003. 63:225-232. [DOD] [CrossRef]
- Torffvit O, Agardh E, Agardh CD. Albuminuria and associated medical risk factors: a cross-sectional study in 451 type II (noninsulin-dependent) diabetic patients. Part 2. J Diabet Complications 1991. 5(1):29-34. [DOD] [CrossRef]
- Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care 1992. 15:815-819. [DOD] [CrossRef]
- Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ 1997. 314:783. [DOD]
- The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 1995. 47(6):1703-1720. [DOD] [CrossRef]
- Levin SR, Coburn JW, Abraira C, Henderson WG, Colwell JA, Emanuele NV, Nuttall FQ, Sawin CT, Comstock JP, Silbert CK. Effect of intensive glycemic control on microalbuminuria in type 2 diabetes. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type 2 Diabetes Feasibility Trial Investigators. Diabetes Care 2000. 23:1478-1485. [DOD] [CrossRef]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000. 321:405-412. [DOD] [CrossRef]
- Forsblom CM, Groop PH, Ekstrand A, Totterman KJ, Sane T, Saloranta C, Groop L. Predictors of progression from normoalbuminuria to microalbuminuria in NIDDM. Diabetes Care 1998. 21:1932-1938. [DOD] [CrossRef]
- Gall MA, Borch-Johnsen K, Hougaard P, Nielsen FS, Parving HH. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes 1995. 44(11):1303-1309. [DOD] [CrossRef]
- Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 1995. 18:258-268. [DOD] [CrossRef]
- Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med 1998. 158:998-1004. [DOD] [CrossRef]
- Schmitz A, Vaeth M, Mogensen CE. Systolic blood pressure relates to the rate of progression of albuminuria in NIDDM. Diabetologia 1994. 37:1251-1258. [DOD] [CrossRef]
- Haneda M, Kikkawa R, Togawa M, Koya D, Kajiwara N, Uzu T, Shigeta Y. High blood pressure is a risk factor for the development of microalbuminuria in Japanese subjects with non-insulin-dependent diabetes mellitus. J Diabetes Complications 1992. 6(3):181-185. [DOD] [CrossRef]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998. 317(7160):703-713. [DOD]
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998. 317(7160):713-720. [DOD]
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993. 329:1456-1462. [DOD] [CrossRef]
- Deferrari G, Ravera M, Deferrari L, Vettoretti S, Ratto E, Parodi D. Renal and cardiovascular protection in type 2 diabetes mellitus: angiotensin II receptor blockers. Am J Soc Nephrol 2002. 13:S224-S229. [DOD] [CrossRef]
- Cruickshanks KJ, Ritter LL, Klein R, Moss SE. The association of microalbuminuria with diabetic retinopathy. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology 1993. 100:862-867. [DOD]
- Agardh CD, Agardh E, Torffvit O. The prognostic value of albuminuria for the development of cardiovascular disease and retinopathy: a 5-year follow-up of 451 patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 1996. 32(1-2):35-44. [DOD] [CrossRef]
- Savage S, Estacio RO, Jeffers B, Schrier RW. Urinary albumin excretion as a predictor of diabetic retinopathy, neuropathy, and cardiovascular disease in NIDDM. Diabetes Care 1996. 19:1243-1248. [DOD] [CrossRef]
This article has been cited by other articles:

|
Microvascular complications and risk factors in patients with type 2 diabetes
Del Canizo Gomez FJ, Fernandez Perez C, Moreno Ruiz I, de Gorospe Perez-Jauregui C, Silveira Rodriguez B, Gonzalez Losada T, Segura Galindo A
Endocrinol Nutr 2011. 58(4):163-168
|
|

|
Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial
Parham M, Amini M, Aminorroaya A, Heidarian E
Rev Diabet Stud 2008. 5(2):102-109
|
|
|