Original Data
| Rev Diabet Stud,
2008,
5(2):102-109 |
DOI 10.1900/RDS.2008.5.102 |
Effect of Zinc Supplementation on Microalbuminuria in Patients With Type 2 Diabetes: A Double Blind, Randomized, Placebo-Controlled, Cross-Over Trial
Mahmoud Parham, Massoud Amini, Ashraf Aminorroaya, Esfandiar Heidarian
Isfahan Endocrine and Metabolism Research Center, Sedigheh Tahereh Research Complex, Isfahan University of Medical Sciences, Isfahan, Iran
Address correspondence to: Ashraf Aminorroaya, e-mail: aminorroaya@med.mui.ac.ir
Manuscript submitted July 7, 2008; resubmitted July 21, 2008; accepted August 8, 2008.
Keywords: type 2 diabetes, microalbuminuria, oxidative stress, diabetic nephropathy, zinc, endothelial cell
Abstract
OBJECTIVES: Oxidative stress can contribute to microvascular complications in diabetes. A decisive event associated with this condition may be the decrease in the synthesis of zinc-containing antioxidant enzymes such as superoxide dismutase and glutathione peroxidase. This consideration led us to investigate the effect of zinc supplementation versus placebo on microalbuminuria in diabetic patients in a randomized double blind clinical trial. METHODS: Fifty diabetic patients with microalbuminuria were enrolled. Fasting plasma glucose, HbA1c, lipid profiles, plasma zinc levels and random urine for albumin and creatinine were measured. Patients randomly received 30 mg elemental zinc (group 1) or placebo (group 2) for 3 months. After a 4 week wash-out period, the groups were crossed over (i.e. the zinc group were given placebo, and the placebo group were given zinc) and the protocol was repeated. RESULTS: From an initial number of 50 selected patients (25 in each of two groups), 39 patients (21 in group 1 and 18 in group 2) completed the study. In group 1, after zinc supplementation, urinary albumin excretion decreased significantly from 86.5 ± 57 to 75 ± 71 mg/g (p = 0.01). After placebo, patients in group 1 showed no significant reduction in microalbuminuria (85 ± 72 mg/g to 83 ± 63 mg/g creatinine). In group 2, no change in albumin excretion was observed after placebo treatment (90.5 ± 63 mg/g to 90 ± 60 mg/g creatinine). After zinc supplementation, a significant reduction was observed in albumin excretion, from 90 ± 60 mg/g to 85 ± 57 mg/g creatinine (p = 0.003). CONCLUSIONS: Zinc supplementation reduced albumin excretion in microalbuminuric type 2 diabetic patients. This outcome may be due to the antioxidant effect of zinc.
Introduction
Renal failure is the most important complication arising from diabetes [1]. It is a major cause of mortality and morbidity in diabetic patients [1, 2]. Microalbumiuria is an early indicator of renal impairment. It is important to identify diabetic patients with this early stage of renal failure in order to set them on medication or diet. In a Japanese population the prevalence of microalbumiuria among type 2 diabetic patients was recently reported to be 31.6% [3]. In an Iranian population, the average incidence rate among type 2 diabetic patients was 82.3/1000 person/year, with a significantly higher incidence for men than for women (104.4 vs. 66.2/1000 person/year, p < 0.001) [4].
There is particular interest in the idea that oxidative stress is relevant in the pathogenesis of diabetic nephropathy. Oxidative stress can be caused either by the increased production of reactive oxygen species (ROS) or a deficiency in antioxidant defenses [5]. Antioxidant deficiency can result from low intake of vitamins, such as vitamins C and E, or impaired synthesis of enzymes, such as superoxide dismutase and glutathione peroxidase, due to zinc deficiency. Zinc is a part of these enzymes' structures and its deficiency may impair their synthesis [5]. Another hypothesis is that hyperglycemia causes endothelial cell dysfunction through a number of pathways, including increased oxidative stress [6, 7] and impaired endothelium-derived relaxing factors such as nitric oxide [8, 9]. In comparison with non-diabetic individuals, diabetic patients have an inferior antioxidant status [5]. Also, diabetes is accompanied by hyperzincuria [10]. The combination of these mechanisms can increase oxidative stress.
Given these considerations, it seems reasonable that antioxidants, such as zinc, might be used to treat or prevent diabetic nephropathy. Unfortunately, there are few studies in this field, which contain some controversial results. In one trial, using a combination of zinc and magnesium, a decreased urinary albumin excretion was observed without any significant effect on blood pressure or lipid profiles [11]. Another study found that intake of zinc and melatonin for three months reduced plasma lipid concentrations and urinary albumin excretion [12]. In yet another trial, reduced HbA1c levels were detected after zinc supplementation [13], whilst elsewhere it was found that HbA1c and lipid profiles remained unchanged after three months of zinc supplementation [14].
Due to these divergent results, further studies are needed to determine more accurately the effect of zinc supplementation on the development of renal disease. This led us to design a randomized placebo-controlled clinical trial to investigate the role of the zinc supplementation versus placebo on microalbuminuria.
Subjects and methods
Subjects
A single-center double blind randomized placebo-controlled crossover clinical trial was performed in Isfahan Endocrine and Metabolic Research Center (IEMRC) between September 2007 and May 2008. All patients were involved in the study for 7 months. Fifty nine type 2 diabetic patients with microalbuminuria (30 mg/g ≤ albumin/creatinine < 300 mg/g) were enrolled by consecutive patient selection. Each patient had microalbuminuria documented in at least two urine samples within six months.
All patients were receiving angiotensin converting enzyme (ACE) inhibitors or angiotensin (AT) receptor blockers for albuminuria. We added zinc to their medication. Demographic data and medication taking were recorded. Afterwards, patients were examined and blood pressure (BP) was measured. Patients were sitting at least for five minutes before measurement of BP [15]. BP was measured twice at ten-minute intervals, before taking any probable antihypertensive medication [15]. It was measured in both arms using mercury sphygmomanometer (Yamasu, Japan); if we observed a disparity in measurement, the higher value was recorded [16].
The day after the first visit, early morning fasting blood and urinary samples were obtained to measure random urine for urinalysis, albumin and creatinine. At the same incidence we measured fasting plasma glucose, serum creatinine, HbA1c, lipid profiles (including total cholesterol, triglyceride, HDL and LDL cholesterol), plasma zinc levels. Also, we did liver function test by measuring serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
Nine patients were excluded after the initial visit because they did not meet the eligibility criteria, yielding a total of 50 patients available for the study. Inclusion and exclusion criteria for the study are shown in Table 1.
Table
1.
Inclusion and exclusion criteria to investigate the effect of zinc versus placebo on microalbuminuria in type 2 diabetic patients |
|
 |
 |
A simple method was used to randomize the study patients. 25 cards were marked 'zinc', and another 25 cards were marked 'placebo'. The cards were shuffled to remove any semblance of order and were placed in a box. After enrollment, each patient was asked to take a card out of the box, under the supervision of an IEMRC staff member. An independent person from IEMRC was given sole responsibility for determining which patients should receive placebo and which should receive zinc in the first phase of the study. All medications (zinc or placebo) rested in the hands of this person. The randomized patient distribution was not disclosed to the research team or to the patients at any time during the study. The codes were destroyed at the end of the study.
In this way, half of the patients (group 1) received 30 mg elemental zinc, and the other half (group 2) received the placebo, each day for 3 months. The zinc sulfate was encapsulated in an oval yellow capsule (Alhavi, Iran). Each capsule contained 132 mg zinc sulfate, amounting to 30 mg elemental zinc. The placebo capsules contained 30 mg of lactose and were made to appear the same as the zinc capsules, in size, shape and color. The prescribed zinc dosage was based on the method used in other studies [11, 14].
Forty five days after treatment (zinc or placebo), patients were visited and interviewed about possible side effects, and to determine the degree of compliance. After 3 months of treatment, at the end of the first phase of the study, all of the laboratory tests and clinical evaluations were repeated as per the initial visit. The study protocol is shown in Figure 1.
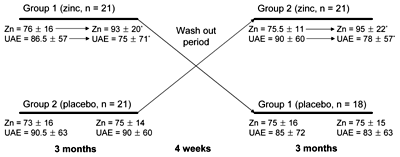 |
 |
Figure 1. Schematic of the study protocol and outcome data. Comparison of urinary albumin excretion and serum zinc levels in type 2 diabetic patients before and after treatment with placebo and zinc in group 1 and group 2 (separately). Group 1 received zinc in the first and placebo in the second phase, group 2 received placebo in the first and zinc in the second phase. Data are mean ± SD at 4 reading points: baseline, after 3 months of treatment, after wash out and after additional 3 months of treatment. Zn: serum zinc level (µg/dl). UAE: urinary albumin excretion (mg/g). * p < 0.05. |
|
After a 4-week wash-out period, the groups were crossed over, so that patients (group 1) initially receiving zinc received the placebo, and patients previously taking the placebo (group 2) now received 30 mg zinc per day. The protocol was repeated again for 3 months. Patient compliance was assessed by capsule counts at each visit, and through measurement of changes in zinc serum levels.
A total of eight patients stopped taking part in the first phase of the study. Two withdrew due to epigastric pain (one patient in each group). Two patients needed a change of drugs (one group 1 patient was put on statins, and one group 2 patient was put on gemfibrozil). Two other patients started with insulin. Furthermore, two group 1 patients were withdrawn for poor compliance.
At the beginning of the second phase of the study, three of the group 1 patients refused to start the second phase. However, as they had completed the first phase, in which they were all taking zinc, we have included their data in the first phase analysis. In total, 39 patients completed both phases of the study. The results and statistics were presented as intentions for treatment.
The IEMRC Medical Ethics Committee approved the study protocol, and all patients gave their written consent. The study complied with the current version of the Declaration of Helsinki.
Laboratory assessments
After 12 hours of overnight fasting, between 8 and 10 a.m., before taking medications, blood and urine samples were collected from all patients. The samples were stored at -70°C at the IEMRC laboratory and assayed at the end of the study.
Urinary albumin concentrations were measured by immunoturbidimetric assay (Pars Azmun kit). Its intra- and inter-assay CV was 1.3% and 2.9%, respectively, and was reported in mg/dl. Urine and serum creatinine was measured using Jaffe reaction [17]. Its intra assay CV was 3.2% and urine results were expressed in g/dl excretion. Microalbuminuria was determined by calculating the ratio of urine albumin to urine creatinine and was expressed in mg/g [18]. The glomerular filtration rate (GFR) was measured using the Cockcroft-Gault equation by calculating (140-age) × (weight in kg)/(72 × serum creatinine in mg/dl) in males, and factored by 0.85 for females [19]. Serum AST and ALT levels were measured by the IFCC method, its intra and inter assay CV was 2.8% and 1.5%, respectively. Normal value for both levels was less than 41 IU/l.
Fasting plasma glucose was measured by the enzymatic method. Its intra- and inter-assay CV was 1.5% and 0.91%, respectively. Serum triglyceride, total cholesterol and HDL were enzymatically measured. Intra- and inter-assay CV for triglyceride was 1.5% and 1.2%, respectively. Intra- and inter-assay CV for HDL was 1.8% and 1.3%, respectively. The values for total cholesterol were 0.95% and 1.1%, respectively. The plasma LDL cholesterol concentration was then calculated by the Friedewald formula [20]: LDL cholesterol = total cholesterol - HDL - VLDL (triglycerides/5) if triglyceride is <400 mg /dl. All biochemical assays were measured by Pars Azmun kits, and BT 300 plus automatic analyzer.
HbA1 was measured by chromatographic method using commercial kit (BioSystem S.A). Its intra- and inter-assay CV was 5.8% and 6.7%. Plasma zinc level was determined by flame atomic absorption spectrometry (spectr AA 220) [21].
Statistical analysis
The power of the study was 80% based on a previously published study [11]. Kolmogorov-Smirnov test was performed to assess the normality of the variables before further statistical analysis. All data in this study are normally distributed, and are presented as mean ± SD.
Zinc or placebo effects for variables in the same individuals were analyzed by paired t test. Data between the two groups were compared using student's t test. Chi-square test was used to compare qualitative variables between the two groups. Multiple regression analysis was used to adjust for the effect of HbA1c on albuminuria. p-values less than 0.05 were considered statistically significant. Statistical analysis was performed by Statistics Package for Social Science (SPSS version 13.0, SPSS Inc., Chicago, IL, USA).
Results
Out of 50 patients selected at the beginning of the study, forty two (84%) completed the first phase. Coincidentally, four patients in each group had withdrawn at the end of the first phase, leaving 21 patients in each group. By the end of the second phase there were 39 (78%) patients remaining i.e. 21 in group 1, and 18 in group 2. As shown in Table 2, at the beginning of the study, basal characteristics were not significantly different between two groups.
Table
2.
Baseline characteristics of the two groups of type 2 diabetic patients with microalbuminuria |
|
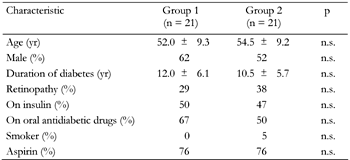 |
 |
Legend:
Data are mean ± SD or percent. n.s.: not significant. |
|
Statistical analysis at the beginning of the study showed that both groups were biochemically and clinically similar, with the exception of serum triglyceride. In particular, urinary albumin excretion was not statistically different between both groups (Table 3).
Table
3.
Baseline biochemical and clinical characteristics of the two groups of type 2 diabetic patients with microalbuminuria |
|
 |
 |
Legend:
Data are mean ± SD. UAE: urinary albumin excretion. FPG: fasting plasma glucose. SBP: systolic blood pressure. DBP: diastolic blood pressure. HDL: high-density lipoprotein. LDL: low-density lipoprotein. n.s.: not significant. |
|
Zinc levels and urinary albumin excretion were compared at baseline and after wash-out period in both groups. The wash-out was carried out for 4 weeks to make sure that it was long enough to eliminate the effects of zinc and placebo before starting the second phase of the study. The results showed that there was no difference between basal parameters at the beginning of first and second phases of the study.
In group 1 (patients who received zinc in the first phase and placebo in the second phase), zinc levels were 76 ± 16 μg/dl at baseline and 75 ± 16 μg/dl at post wash-out period (p = 0.4). Urinary albumin excretion in this group was 86.5 ± 62 mg/g at baseline and 85 ± 72 mg/g at post wash-out period (p = 0.2). In group 2 (patients who received placebo in first phase and zinc in second phase), zinc levels were 73 ± 16 μg/dl and 75.5 ± 11 μg/dl at baseline and post wash-out period, respectively (p = 0.4). Urinary albumin excretion in this group was 90.5 ± 63 mg/g and 90 ± 60 mg/g at baseline and post wash-out period, respectively (p = 0.9; Figure 1).
At baseline, 4 patients in group 1 and 4 in group 2 had low concentrations of serum zinc. Zinc was considered to be deficient when serum zinc level was <60 μg/dl [22]. GFR was not altered during zinc supplementation or placebo compared with baseline (Tables 4 and 5).
Table
4.
Comparison of blood pressure and biochemical characteristics in type 2 diabetic patients before and after treatment with zinc (first phase) and placebo (second phase) in group 1 |
|
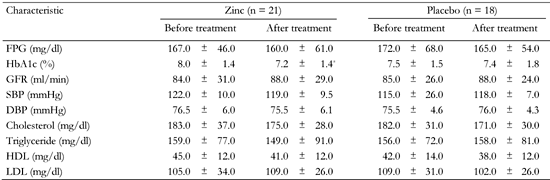 |
 |
Legend:
Data are mean ± SD. FPG: fasting plasma glucose. GFR: glomerular filtration rate. SBP: systolic blood pressure. DBP: diastolic blood pressure. HDL: high-density lipoprotein. LDL: low-density lipoprotein. * p < 0.05. |
|
Table
5.
Comparison of blood pressure and biochemical characteristics in type 2 diabetic patients before and after treatment with placebo (first phase) and zinc (second phase) in group 2 |
|
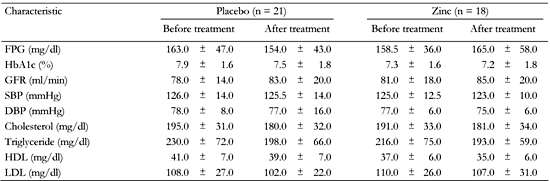 |
 |
Legend:
Data are mean ± SD. FPG: fasting plasma glucose. GFR: glomerular filtration rate. SBP: systolic blood pressure. DBP: diastolic blood pressure. HDL: high-density lipoprotein. LDL: low-density lipoprotein. |
|
Effects on serum zinc level and microalbuminuria
Compared with baseline (p = 0.001 in both groups), serum zinc levels increased significantly in both groups after zinc supplementation.. As expected, no significant changes in zinc levels were observed after placebo administration (p = 0.6 for group 1, and p = 0.2 for group 2; Figure 1).
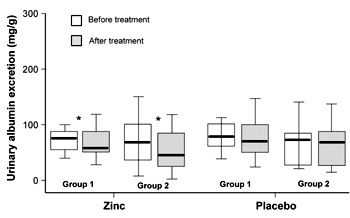
In comparison with baseline after 3 months of zinc supplementation, urinary albumin excretion decreased significantly (p = 0.01 in group 1 and p = 0.003 in group 2; Figures 1 and 2). After 3 months of placebo intake, there were no significant changes in urinary albumin excretion compared with baselines in both groups (Figures 1 and 2).
 |
 |
Figure 2. Comparison of urinary albumin excretion in type 2 diabetic patients before and after treatment with zinc or placebo in group 1 and group 2. Group 1 received zinc in the first and placebo in the second phase, group 2 received placebo in the first and zinc in the second phase. Rectangle, lower part: 25th percentile. Rectangle, upper part: 75th percentile. Line below rectangle: smallest value that is not an outlier. Line above rectangle: largest value that is not an outlier. * p < 0.05. |
|
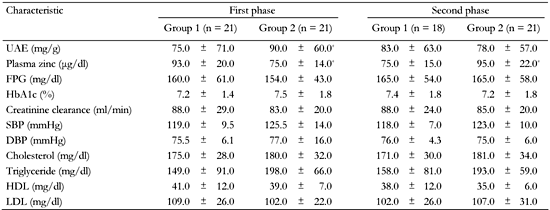
We also compared the two groups at the end of the first and at the end of the second phase. In the first phase, where patients in groups 1 received zinc and in group 2 placebo, differences in plasma zinc levels and urinary albumin excretion between both groups were statistically significant (zinc: 93 ± 20 μg/dl vs. 75 ± 14 μg/dl; urinary albumin excretion: 75 ± 71 mg/g vs. 90 ± 60 mg/g; p < 0.05). This confirms the positive effect of zinc. In the second-phase of the study, when patients in the groups switched treatment, only plasma zinc level but not urinary albumin excretion were significantly different between two groups (Table 6).
Table
6.
Comparison of albuminuria, plasma zinc level, blood pressure and biochemical parameters between type 2 diabetic patients in group 1 and group 2 at the end of each of the two study phases |
|
 |
 |
Legend:
Data are mean ± SD. UAE: urinary albumin excretion. FPG: fasting plasma glucose. SBP: systolic blood pressure. DBP: diastolic blood pressure. HDL: high-density lipoprotein. LDL: low-density lipoprotein. * p < 0.05. |
|
Effects on fasting plasma glucose and HbA1c
Fasting plasma glucose levels were not altered after 3 months of zinc supplementation or placebo intake in either group (Tables 4 and 5). After 3 months of zinc supplementation, HbA1c was reduced significantly in group 1 and remained unaltered in group 2, when compared with baseline over the study period. Analysis using general linear model (ANCOVA), showed that the effect of zinc on decreasing urine albumin excretion was independent of the change in HbA1c (p = 0.03).
Effects on blood pressure
The mean values of systolic and diastolic blood pressure after 3 months of zinc supplementation or placebo were not significantly different from baseline (Tables 4 and 5).
Effects on plasma lipids
After 3 months of zinc supplementation, mean serum levels of total cholesterol, triglyceride, HDL and LDL cholesterol, were not changed in either group compared with baseline (Tables 3 and 4). Also, when they received placebo, no significant changes were observed in the mean values of serum lipid profiles between the two groups in comparison with baseline (Tables 4 and 5).
Discussion
The main purpose of this study was to investigate the effect of zinc supplementation on microalbuminuria. However, other variables such as hyperglycemia, hypertension and hyperlipidemia may affect albumin excretion. Therefore, we included these variables in the study [11, 23]. Our results showed that zinc supplementation decreases microalbuminuria in type 2 diabetic patients. This effect seems to be due to the antioxidant property of zinc. Serum zinc levels increased significantly in both groups when compared with baseline, but this effect was observed only after zinc supplementation. It was not increased after placebo. This result was expected, and showed good compliance of patients with the treatment.
The present study showed that 3 months of zinc supplementation significantly reduced urinary albumin excretion, a marker for renal function. This finding was observed in both groups during zinc supplementation and it may be due to the antioxidant effect of zinc. Our findings are consistent with results of other recently published studies, in which the urinary albumin excretion ratio was seen to be decreased in diabetic patients who had received 30 mg zinc and magnesium [11] or zinc and melatonin for 3 months [12].
Similar to another study, the mean levels of fasting plasma glucose were not significantly changed in either group during zinc or placebo intake (Tables 4 and 5) [11]. In contrast to some studies [11, 12], but in agreement with at least one other [13], there were no significant changes in HbA1c in either group when they received placebo. However, we found a reduction in HbA1c in one zinc treated group. Zinc supplementation decreased HbA1c with no effect on fasting plasma glucose (Tables 4 and 5). It might have also decreased postprandial plasma glucose concentration, which was not measured in our study. On the other hand, zinc may inhibit oxidation of hemoglobin despite elevated blood glucose, due to its antioxidant effect.
The role of intensive glycemic control on microalbuminuria in diabetic patients remains controversial. However, one study has shown that poor glycemic control may be associated with the development of overt proteinuria in type 2 diabetic patients with microalbuminuria [24]. Our analysis using a general linear model showed that the effect of zinc on decreasing urine albumin excretion was independent of the change in HbA1c. Therefore, beneficial effects of zinc on microalbuminuria, independent of glycemic control, suggests that zinc has a renoprotective effect, putatively via its antioxidant property. It would be useful to undertake further study to discover more about the mechanism and the effect that zinc has on the function of alpha- and beta-cells, and on insulin resistance.
Blood pressure was not observed to change during the study period. As hypertension is a potent risk factor for microalbuminuria [25], it seems that the reduction of albumin excretion is independent of changes in blood pressure (Tables 4 and 5).
In accordance to previously published studies [11, 14], we found no significant changes in serum lipid levels. However, in another report, serum lipid concentrations decreased after zinc supplementation [12]. In patients with type 2 diabetes, hyperlipidemia is a risk factor for the progression of nephropathy [26]. Therefore, our findings suggest that zinc does not decrease microalbuminuria through the reduction of lipid profiles.
A limitation of our study may be that we did not measure C-reactive protein (CRP) as an inflammatory factor, which may affect microalbuminuria. However, the correlation between CRP and albuminuria has already been reported to be low [27]. Zinc may also affect the alpha-cell function. However, it was not the main purpose of this study to detect the effect of zinc on islet function and glucagon levels. We recommend that this mechanism is investigated in future studies.
Acknowledgments:
The authors would like to thank Ms. Zaree and Ms. Akbary for organizing a team to invite people to take part and give feedback. Also our thanks to Mr. Abyar who helped in computer affairs of the research.
References
- Rosen P, Toeller M. Vitamin E in diabetes: increased oxidative stress and its prevention as a strategy to prevent vascular complications. Int J Vitam Nutr Res 1999. 69:206-212. [DOD] [CrossRef]
- Nakamura T, Ushiyama C, Suzuki S, Shimada N, Ohmuro H, Ebihara I. Effects of taurine and vitamin E on microalbuminuria, plasma metalloproteinase-9, and serum type IV collagen concentrations in patients with diabetic nephropathy. Nephron 1999. 83:361-362. [DOD] [CrossRef]
- Yokoyama H, Kawai K, Kobayashi M. Microalbaminuria is common in Japanese type 2 diabetic patients. Diabetes Care 2007. 30 (4):989-998. [DOD] [CrossRef]
- Amini M, Safaei H, Aminorroaya A. The incidence of microalbuminuria and its associated risk factors in type 2 diabetic patients in Isfahan, Iran. Rev Diabet Stud 2007. 4(4):242-248. [DOD] [CrossRef]
- Kelly FJ. Use of antioxidants in the prevention and treatment of disease. J Int Fed Clin Chem 1998. 10(1):21-23. [DOD]
- Baynes JW, Thorpe SR. The role of oxidative stress in diabetic complications. Curropin Endocrinol 1996. 3:277-284. [DOD]
- Giugliano D, Cerellio A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996. 19:257-265. [DOD] [CrossRef]
- Elliott TG, Cockcroft JR, Groop PH, Viberti GC, Ritter JM. Inhibition of nitric oxide synthesis in forearm vasculature of insulin-dependent diabetic patients: blunted vasoconstriction in patients with microalbuminuria. Clin Sci 1993. 85:687-693. [DOD]
- Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardio 1996. 27(3):567-574. [DOD] [CrossRef]
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 2006. 20(1):3-18. [DOD] [CrossRef]
- Farvid MS, Jalali M, Siassi F, Hosseini M. Comparison of the effects of vitamins and/or mineral supplementation on glumerular and tubular dysfunction in type 2 diabetes. Diabetes Care 2005. 28(10):2458-2464. [DOD] [CrossRef]
- Kadhim HM, Ismail SH, Hussein KI, Bakir IH, Sahib AS, Khalaf BH, Hussain SA. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J Pineal Res 2006. 41(2):189-193. [DOD] [CrossRef]
- Al-Maroof RA, Al-Sharbatti SS. Serum zinc level in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetes. Saudi Med J 2006. 27(3):344-350. [DOD]
- Roussel AM, Kerkeni A, Zouari N, Mahjoub S, Matheau JM, Anderson RA. Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J Am Coll Nutr 2003. 22:316-321. [DOD]
- O'Brien E, Asmar R, Beilin L. Practice guidelines of the European Society of hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 2005. 23:697-702. [DOD]
- Gosse P. Blood pressure should be measured in both arms on the first consultation. J Hypertens 2002. 20:1045-1055. [DOD] [CrossRef]
- Stol C. Plasma creatinine determination: a new and specific Jaffe reaction method. Scand J Clin Lab Invest 1965. 17:381-387. [DOD] [CrossRef]
- Mogensen CE, Keane WF, Bennett PH. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet 1995. 346:1080-1084. [DOD] [CrossRef]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976. 16:31-36. [DOD] [CrossRef]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of LDL in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972. 18:499-502. [DOD]
- Arnaud J, Bellanger J, Bienvenu F, Chappuis P, Favier A. Recommended method for assaying serum zinc with flame atomic absorption. Ann Biol Clin 1986. 44:77-87. [DOD]
- Hambidge KM, Casey CE, Krebs NF. Zinc. In: Mertz W (editor). Trace elements in human and animal nutrition. 5th ed., Academic Press, New York, 1986, p. 1-10. [DOD]
- Hasslacher C, Bostedt-Kiesel A, Kempe HP, Wahl P. Effect of metabolic factors and blood pressure on kidney function in proteinuric type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993. 36:1051-1056. [DOD] [CrossRef]
- Song KH, Yoon KH, Kang ML, Cha BY, Lee KW, Son HY, Kang SK. Progression to overt proteinuria in microalbuminuric Koreans with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 1998. 42(2):117-121. [DOD] [CrossRef]
- Frohlich ED, Arthus C. Influence of nitric oxide and angiotensin II on renal involvement in hypertension. Hypertension 1997. 29:188-193. [DOD]
- Wirta OR, Pasternack AI, Mustonen JT, Koivula TA, Harmoinen A. Urinary albumin excretion rate and its determinants after 6 years in non-insulin-dependent diabetic patients. Nephrol Dial Transplant 1996. 11:449-456. [DOD]
- Bakker S, Gansevoort R, Stuveling EM, Gans R, Zeeuw D. Microalbuminuria and C-reactive protein: similar messengers of cardiovascular risk? Curr Hypertens Rep 2005. 7:379-384. [DOD]
This article has been cited by other articles:

|
Metal-based anti-diabetic drugs: advances and challenges
Levina A, Lay PA
Dalton Trans 2011. In press
|
|

|
Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome
Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, Poursafa P, Rouzbahani A
Metab Syndr Relat Disord 2010. 8(6):505-510
|
|

|
Zinc and zinc transporter regulation in pancreatic islets and the potential role of zinc in islet transplantation
Bosco MD, Mohanasundaram DM, Drogemuller CJ, Lang CJ, Zalewski PD, Coates PT
Rev Diabet Stud 2010. 7(4):263-274
|
|

|
Diabetes and vascular disease: basic concepts of nitric oxide physiology, endothelial dysfunction, oxidative stress and therapeutic possibilities
Capellini VK, Celotto AC, Baldo CF, Olivon VC, Viaro F, Rodrigues AJ, Evora PR
Curr Vasc Pharmacol 2010. 8(4):526-544
|
|

|
Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus
Foster M, Samman S
Antioxid Redox Signal 2010. 13(10):1549-1573
|
|

|
Zinc supplementation partially prevents renal pathological changes in diabetic rats
Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Jin L, Xiao J, Xie R, Rane M, Li X, Cai L
J Nutr Biochem 2010. 21(3):237-246
|
|

|
Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children
Hashemipour M, Kelishadi R, Shapouri J, Sarrafzadegan N, Amini M, Tavakoli N, Movahedian-Attar A, Mirmoghtadaee P, Poursafa P
Hormones 2009. 8(4):279-285
|
|

|
Effect of zinc supplementation on serum homocysteine in type 2 diabetic patients with microalbuminuria
Heidarian E, Amini M, Parham M, Aminorroaya A
Rev Diabet Stud 2009. 6(1):64-70
|
|
|