Editorial
| Rev Diabet Stud,
2008,
5(3):128-135 |
DOI 10.1900/RDS.2008.5.128 |
The Role of Surrogate Endpoints in the Evaluation of Efficacy and Safety of Therapeutic Interventions in Diabetes Mellitus
Aleksandra Wieczorek1, Przemyslaw Rys1, Iwona Skrzekowska-Baran2, Maciej Malecki3
1HTA Consulting, Krakow
2NovoNordisk Polska
3Department of Metabolic Diseases, Jagiellonian University College of Medicine, Krakow, Poland
Address correspondence to: Maciej Malecki, e-mail: mmalecki@cm-uj.krakow.pl
Manuscript submitted October 26, 2008; resubmitted November 21, 2008; accepted November 23, 2008.
Keywords: diabetes, patient-important endpoint, clinical study, metformin, HbA1c, metabolic control
Abstract
In this paper, we examine the concept of surrogate endpoints (i.e. substitute outcome measures) and review their use in clinical trials involving therapies for diabetes mellitus using the example of metformin. Trials such as DCCT and UKPDS, in which patient-important endpoints were evaluated, are relatively rare in diabetology. Clinical decisions, therefore, are often based on evidence obtained using surrogate outcomes, usually fasting or postprandial glycemia or glycated hemoglobin level. In contrast to patient-important endpoints, surrogates do not describe direct clinical benefit to the patient. However, a proven association between a surrogate and patient-important endpoint is essential to draw appropriate therapeutic conclusions. In the process of new drug development, the duration of follow-up, sample size and methodology of the studies initially available are often inadequate to demonstrate the effect of the intervention on patient-important endpoints. Evidence concerning the effect of an intervention on surrogate outcomes usually comes first, followed only later by reports describing its influence on patient-important endpoints. Metformin may serve as an example in several ways. The first publications reported beneficial effects on glycemic control and body weight. Outcomes from the subsequent UKPDS study suggested the patient-important efficacy of metformin measured as a reduction in mortality and a decrease in the incidence of diabetic complications, including myocardial infarction. This reasoning process worked for some but not all strategies. It is particularly questionable whether a change in surrogate endpoint was associated with a potential deterioration in patient-important outcomes. Defining the general relationship between surrogates widely used as measures of metabolic control and patient-important endpoints remains an important challenge in contemporary diabetology.
Vocabulary of endpoints description in clinical trials
Understanding and interpreting the methodology and results of clinical studies requires familiarity with a wide range of terms and definitions which, if not used carefully, may be difficult or confusing for the reader of clinical literature. The different concepts associated with describing an endpoint may serve as an example. An endpoint (or an outcome) is any measurable effect (usually, but not necessarily, related to health) observed in individuals participating in a clinical trial. The effect of an external factor (for example, tobacco smoking as predictive factor or a prophylactic cholesterol lowering drug as therapeutic factor) on this kind of selected endpoint is investigated in clinical studies [1]. Readers of clinical literature may encounter different descriptions of outcomes/endpoints: primary versus secondary, hard versus soft, clinically important, patient-important and surrogate.
The issue of primary and secondary endpoints is one of the least complex; it refers to the investigators’ selection of measurements prior to the study, a choice influencing their sample size calculations, analysis and reporting of results. In some trials, the primary endpoint may be mortality or stroke, in others resource use and in other studies the average change of cholesterol level, creatinine level, blood pressure, or number of hairs per cm2. Those outcomes may or may not be objective (hard, meaning reproducible and not influenced by the measurement process, i.e. death, amputated limb, cholesterol level) versus subjective (soft, meaning potentially influenced by the measurement process or with questionable reproducibility, i.e. description of the patient’s mood at a given moment).
In comparison to the distinction between endpoints made above (hard versus soft, primary versus secondary), the difference between clinically important or patient-important versus surrogate (or substitute) endpoints is sometimes less evident. One of the ways to define patient-important outcomes is to ask ourselves and our patients a question, namely if this was the only outcome changed by the given intervention would it be worth using that treatment (even assuming that it was non-toxic and not excessively expensive). Mortality, functional capacity, myocardial infarction, well-being, symptoms of hyper- or hypoglycemia are likely to fulfill this criterion; cholesterol level, HbA1c or blood pressure lowering effects (again, in the absence of other reasonably proven benefit) most likely do not. This in no way implies that treating hypertension or hypercholesterolemia is not worthwhile, as these parameters are still the therapeutic goal, but it is important to remember that some lipid or cholesterol-lowering drugs may have more impact on patient-important outcomes than others and some of them may even be harmful [2]. Clinically important, clinically relevant, patient-oriented and patient-important endpoints are frequently used interchangeably, and what is more important in the context of this article, are used in contrast to surrogate endpoint. Our language will reflect our preference to define what is clinically important from the patient’s perspective using, whenever possible, the concept and phrase ‘patient-important’ endpoint as opposed to surrogate endpoint [3].
According to the current definition of the Food and Drug Administration (FDA), a surrogate endpoint is a substitute for a patient-important endpoint, i.e. substitute for a therapeutic effect [4, 5]. In this sense, surrogate endpoints (also sometimes called substitute or intermediate endpoints) are those which are not directly patient-important, yet are considered to be important or at least relevant in the decision-making process.
The first statistical definition of a surrogate was formulated by Prentice in 1989 [6]. He defined a surrogate endpoint as “a response variable, for which a test of the null hypothesis of no relationship to the treatment groups under comparison is also a valid test of the corresponding null hypothesis based on the “true” endpoint”. Such a definition requires that the studied intervention has a statistically significant effect on both patient-important and surrogate endpoints and that the entire effect of treatment on the “true” endpoint is conveyed by the surrogate [6]. The latter condition, when included in the first definition of a surrogate endpoint, was considered too restrictive [7]. For example, ACE inhibitors and calcium blockers have similar impact on blood pressure lowering but their influence on cardiovascular events is different, which may mean that blood pressure control is not the only factor responsible for treatment effect [8-10]. Many authors, including Busye, Molenbreghs and Friedman [7, 11, 12], made a significant contribution to making this definition more practical. The core of surrogate use is belief in their association (sometimes casual) with patient-important outcome.
Validation is a term commonly used in the context of surrogate assessment. It may be used as statistical validation (underlining statistical correlation between the investigated phenomena) or as biological considerations (in which a causal relationship is stressed). This group of endpoints includes biochemical (e.g. serum cholesterol level) as well as pathophysiological (e.g. arterial blood pressure) or morphological variables (such as the diameter of a coronary artery or left ventricular hypertrophy). Some authors accept that surrogates may not be directly involved in the pathophysiology of the disease but reflect the activity of a process leading to unfavorable events [13]. An example of the latter could be the level of changes in HbA1c. Glycation of hemoglobin per se does not cause severe pathophysiological consequences; however, its intensity reflects glycemic exposure over a period of a few months.
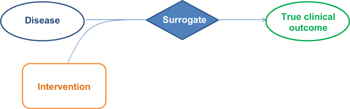
To summarize, a surrogate does not describe direct benefit to the patient and its usefulness in evaluating such benefit depends on how much it allows for prediction of treatment effect on patient-important outcomes (Figure 1). A parameter that is potentially useful as a surrogate endpoint may be:
- directly involved in the pathophysiological pathway of the disease (i.e. it is identical with one of the mechanisms, on which a medication or intervention used in treatment of the disease acts), for example coronary artery stenosis as a surrogate for myocardial infarction or cardiac death;
- related to, but not directly involved in, the pathophysiology of the disease (i.e. directly associated with one of the stages of the disease occurrence and thus correlating with its progression or response to treatment), for example serum cholesterol level as a biomarker of myocardial infarction risk;
- not directly associated with any disease mechanism or its response to treatment (for example HbA1c level as a surrogate for the incidence of late complications of diabetes mellitus)[14].
 |
 |
Figure 1. The relationship between surrogates and true clinical outcomes. |
|
It is sometimes neither possible nor feasible to perform a valid evaluation of the efficacy and safety of the treatment based on patient-important endpoints in a reasonably short time. Trials, in which those clinically relevant endpoints are evaluated, require a relatively long time and large investigated groups. These conditions complicate the organization and increase the costs of such trials, but the most important problem is the time usually required before the results are known, a period usually measured in years. Meanwhile, patients and physicians expect new medications to be introduced onto the market as rapidly as possible.
Therapeutic decisions based exclusively on surrogate endpoints have their advantages and disadvantages. On the one hand, such decisions may result in the quicker introduction of a specific intervention into clinical practice, as was the case with certain antiviral drugs used in HIV infection therapy, where the decision was based on their ability to increase CD4+ cell count [15]. Such a policy also makes it possible to reduce the costs of assessing a health technology or medical product. On the other hand, in certain situations this may lead to false conclusions resulting in unfavorable clinical consequences. For example, sodium fluoride was proven to increase bone mineral density (BMD), but, contrary to expectations, clinical trials demonstrated that it had no effect on the fracture rate in postmenopausal women [16]. Worse still, certain antiarrhythmic drugs were introduced on the basis that they suppressed premature ventricular contractions with the hope of saving lives. The results of clinical trials looking at patient-important outcomes proved this supposition to be tragically wrong [17]. Use of surrogates is therefore hampered by the fact that the evaluation of their association with relevant clinical endpoints may be incomplete, inadequate or downright erroneous.
Surrogate endpoints in diabetes mellitus trials
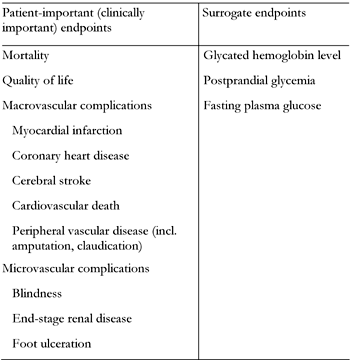
The surrogate endpoints most often used in diabetes mellitus studies include HbA1c level, FPG and PPG. The patient-important and surrogate endpoints related to diabetes mellitus are summarized in Table 1. It is interesting to note that some stages of a particular diabetic complication, for example nephropathy, should be considered patient-important while others tend to be classified as a substitute (end-stage renal disease vs. microalbuminuria) [18]. The pathophysiological rationale for the use of HbA1c, FPG and PPG in assessment of metabolic control in diabetes mellitus is well documented. However, this does not automatically mean that any therapeutic intervention resulting in improved metabolic control as assessed by these parameters reduces the risk of developing late micro- and macrovascular complications or of patients’ mortality. According to recommendations from the American Diabetes Association (ADA), World Health Organization (WHO) and American College of Endocrinologists (ACE), HbA1c level is considered the “gold standard” in assessment of metabolic control and the specific level of HbA1c constitutes the target at which treatment of both type 1 and 2 diabetes mellitus should be aimed [19]. It should be noted that in two fundamental diabetology studies, the DCCT in type 1 and the UKPDS in type 2 diabetes mellitus, pharmacological interventions leading to the reduction of this parameter were associated with improvement in clinical endpoints, i.e. microvascular complications [20, 21].
Table
1.
Comparison of endpoints used in diabetes mellitus clinical trials |
|
 |
 |
All three organizations (ADA, WHO and ACE) also underline the importance of normalizing FPG and PPG. Nevertheless, it must be stressed that clinical trials convincingly demonstrating a direct relationship between reduction in PPG and improvement in patient-important endpoints are not yet available. Several trials in patients with type 1 or type 2 diabetes demonstrated the favorable effect of PPG reduction on the intima-media complex thickness (IMT) [22-24]. IMT cannot, however, be considered to be a clear patient-important endpoint, although its correlation with the incidence of cardiovascular events (stroke, myocardial infarction and others) has been described in several studies that included non-diabetic subjects [25-27].
The relationships between the different surrogates reflecting metabolic control in diabetes mellitus are themselves worth consideration. The level of HbA1c alone does not convey comprehensive information about the amplitude and frequency of glycemic fluctuations, especially in patients with type 1 diabetes mellitus, which makes it an insufficient sole marker of glycemic control [28]. A series of trials was undertaken to investigate the relationships between HbA1c level and FPG as well as PPG. Earlier studies demonstrated a weaker correlation of HbA1c with PPG than with FPG [28]. Other reports indicated that relationships between HbA1c level and PPG and FPG may vary, depending on level of metabolic control. A study in patients with type 1 diabetes mellitus demonstrated that PPG in patients whose HbA1c level was in the lowest quartile (<7.3%) was responsible for 70% of its increase above normal levels, while for those in the highest quartile (≥9.3%) the influence of FPG was decisive. Between those two extremes, the effects of FPG and PPG on HbA1c level were similar [29, 30]. These findings were confirmed in the latest research published by Woerle et al. [31]. They showed that the impact of PPG was about 80% on HbA1c when HbA1c was below 6.2%, and only about 40% when HbA1c was >9%.
In many diseases, establishing a relationship between surrogates and patient-important endpoints remains a challenge. Providing such proof may bring benefit to both patients and physicians, as both are interested in finding the most effective therapy. Introducing a new intervention into practice may be much faster if the effect of the intervention on a surrogate is measured and the relationship between the surrogate and a patient-important endpoint is unequivocally proven.
In case of diabetes mellitus, less than twenty large randomized clinical trials evaluating patient-important endpoints have been published. Frequently, there are no clinical trials regarding patient-important endpoints for some widely used interventions; for some interventions, trials of this kind appeared years after the intervention was introduced onto the market.
Could, or what is more important, should the results of clinical trials designed to evaluate surrogate endpoints be used in formulating clinical decisions? Metformin may serve as an example of a medication that was used in diabetic clinical practice for many years despite lack of proven beneficial effect on patient-important outcomes.
The example of metformin – surrogates and patient-important endpoints
Metformin belongs to a group of drugs called biguanids. It is currently one of the most popular medications used in treatment of type 2 diabetes mellitus. It has been in use for more than 50 years [32]. Metformin was banned in USA in 1977 and was removed from US market for the subsequent 2 decades because of uncertainty as to whether it was associated with lactic acidosis. The drug reappeared on the US pharmaceutical market in mid-1990s [33].
In the nineties, use of this drug was restricted by numerous precautions, not only because of the risk of adverse effects, but also because the efficacy of metformin was proven with respect to typical surrogates only. The situation changed dramatically with the publication of the results of the UKPDS study in 1998 [20]. It may be interesting, therefore, to look at the evidence available prior to publication of the UKPDS results and afterwards.
In 1999 a meta-analysis of the results of all studies published up until 1995 where metformin was compared with placebo or sulphonylurea derivatives was published by Johansen [34]. This meta-analysis was based on a valid systematic review of clinical trials, and its results may be considered reliable and up-to-date at the time of publication. A review of the literature published before the mid-1990s identified 9 randomized controlled clinical trials, in which metformin was compared to placebo, and 10 randomized trials, in which metformin was evaluated in comparison with the sulphonylureas. The results of these studies were included in the meta-analysis.
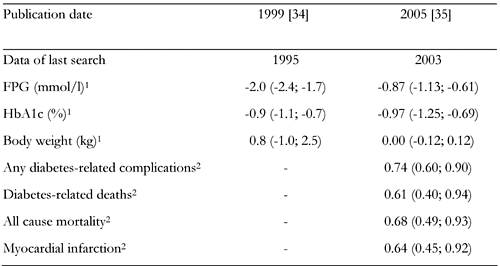
The pooled results of the 9 studies led to the conclusion that metformin, in comparison to placebo, decreased FPG and the HbA1c level and caused no changes in body weight. The specific data are shown in Table 2.
Table
2.
Two meta-analyses on the comparison of metformin vs. placebo in type 2 diabetes mellitus |
|
 |
 |
Legend:
1 Metformin compared with placebo. Data are weighted mean difference (95% CI in parentheses). 2 Metformin compared with conventional therapy. Data are relative risk (95% CI in parentheses). |
|
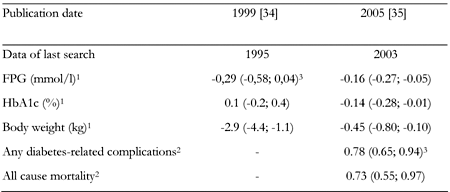
In the same meta-analysis of 10 randomized controlled trials, no differences were found between metformin and sulphonylureas with respect to the change in glycated hemoglobin level or fasting glycemia. At the same time, it was demonstrated that the drugs investigated had the opposite effects on body weight. Use of metformin contributed to reduction of body weight, while use of sulphonylureas increased it (Table 3). The meta-analysis of Johansen, published before the UKPDS study results were available, demonstrated the efficacy of metformin, against placebo, measured by laboratory parameters and suggested a lack of unfavorable effect on body weight. As compared to sulphonylureas, metformin demonstrated similar effects on metabolic control, but its influence on body weight was more favorable.
Table
3.
Two meta-analyses on the comparison of metformin vs. sulphonylureas in type 2 diabetes mellitus |
|
 |
 |
Legend:
1 Metformin compared with sulphonylurea (SU). Data are weighted mean difference (95% CI in parentheses). 2 Metformin compared with SU or insulin. Data are relative risk (95% CI in parentheses). 3 Approximate data, read from the graph. |
|
The UKPDS study results published in 1998 evaluated the effect of metabolic control on clinical outcome in type 2 diabetes mellitus and demonstrated that the risk of death or diabetes-related complications was lower in the group of obese patients treated with metformin as compared to such patients treated with insulin or sulphonylurea derivatives. At the same time, patients treated with metformin (as compared to conventional treatment) were at lower risk of diabetes-related complications, myocardial infarction, cardiovascular death and death regardless of the cause [20].
In 2005, Saenz et al. [35] published a Cochrane Collaboration systematic review, including (among others) the UKPDS study results. In this paper, the favorable effect of metformin versus placebo on metabolic control was confirmed (Table 2). Similarly to Johansen’s meta-analysis [34], no differences between metformin and placebo were demonstrated with respect to changes in body weight (Table 3) [35].
In addition, this meta-analysis demonstrated more favorable effect of metformin on metabolic control in comparison to those of sulphonylureas. The difference between the two drugs was small but statistically significant. It also confirmed that metformin had a more favorable influence on changes in body weight. Most importantly, however, the UKPDS study results proved its benefits with respect to patient-important endpoints (Table 2 and 3) [35].
The above theoretical and historical considerations on the role and position of metformin demonstrate that, in this case, the favorable effects of the drug with respect to surrogates and body weight were confirmed in randomized clinical trials using patient-important outcome measures, long after the drug was introduced on the basis of its effect on metabolic surrogates.
Further comments on surrogate endpoints, epidemiological studies and other classes of diabetic drugs
Our review of the literature indicates that clinical trials using patient-important endpoints are scarce in diabetology. The Cochrane Collaboration, an organization which develops valid and up-to-date systematic reviews, has published dozens of these on diabetes mellitus. Analysis of these and other credible systematic reviews dealing with diabetic management indicates that the efficacy of most interventions, both pharmacological and behavioral, is proven with respect to surrogates, while the effects of those interventions on patient-important endpoints remain unknown or unproven. For example, no studies unequivocally confirming the favorable effect of sulphonylureas on the risk of diabetes-related complications have been published so far. Although sulphonylurea derivatives are used as comparators for new drugs (e.g. the ADOPT and RECORD studies for rosiglitazone) [36, 37, 38], the effect of this group of drugs on primary endpoints as compared to placebo has not been demonstrated [39]. In June 2008, the results of the ADVANCE study, which indirectly suggested that gliclazide has a favorable effect on a combination of macrovascular and microvascular endpoints (or, at least, on new or worsening nephropathy), was published. However, the objective of that study was to assess the effect of strict glycemic control (target HbA1c level ≤ 6.5%) not that of the drug itself on reducing the risk of diabetic complications and, therefore, any conclusions regarding sulphonylureas are indirect [40].
Other anti-diabetic medications, such as alpha-glucosidase inhibitors or meglitidines, were also proven to have a favorable effect on metabolic control, while their effect on patient-important endpoints in type 2 diabetes mellitus remains unknown, unproven or disputable [41, 42]. In 2004, Hanefeld et al. published a meta-analysis [43] which indicated that acarbose exerts a favorable effect on reduction of the risk of cardiovascular complications in type 2 diabetes mellitus; however, that analysis was not based on systematic review. The criteria for considering studies were not clearly specified, only trials from Bayer Acarbose clinical database were included (no systematic searching), no critical appraisal of included studies were performed and only pooled results of the meta-analysis were presented but not data from single studies [44, 45]. However, a systematic review concerning acarbose published by the Cochrane Collaboration one year later [41], failed to confirm the optimistic report made by Hanefeld et al. Publication of the recent ACCORD study casts more doubt on the connection of surrogate endpoint HbA1c (6.4% versus 7.5%) and patient-important outcomes, as the study was interrupted because of excessive mortality in the intensively treated group [46].
Final comments and our conclusions
The facts discussed above indicate that, when dealing with diabetes mellitus, individual physicians, opinion leaders and regulatory bodies have often based (and still frequently base) their decisions or recommendations on the results of clinical trials evaluating surrogate endpoints. Such decisions, albeit difficult and associated with the risk of erroneous clinical reasoning, were made in the past, as illustrated by the example of metformin. Studies evaluating the effect of drugs on metabolic control predominate in diabetology, which is why it was so important that, in pivotal trials, surrogates were found to be associated with patient-important endpoints, at least in the form of microvascular complications and in relation to specific drugs. In the UKPDS study, the relationship between lower HbA1c and a lower risk of developing microvascular complications in type 2 diabetes mellitus was demonstrated. The DCCT study confirmed the favorable effect of metabolic control as measured by this surrogate endpoint on reducing the risk of microangiopathies in type 1 diabetes mellitus.
Additional support for the relationship between surrogate and patient-important outcomes was provided by Selvin et al. [47], who demonstrated in a meta-analysis of observational studies that a 1 percentage point increase in HbA1c level is related to a statistically significant increase in the risk of cardiovascular complications. Along the same lines, Groeneveld et al. reviewing the literature demonstrated that in 23 out of 27 identified studies there was a relatively weak but statistically significant relationship between the metabolic control level (measured using FPG or HbA1c) and mortality [48]. However, it is impossible to ignore opposite findings, including the potential for increased mortality observed in the ACCORD trial. Another finding that cannot be ignored is that from other areas of medicine, where correlation between surrogates and patient-important outcomes hold for some but not all drugs influencing any given surrogate [2].
Considering all of the above, we conclude that both pivotal clinical studies as well as meta-analyses (which are scarce and suffer from limitations) indicate a correlation and association between the improvement in HbA1c level and changes in certain selected patient-important endpoints, best proven for micro-vascular complications. Evidence for the relationship between PPG—used as a surrogate in clinical trials—and the occurrence of patient-important endpoints is lacking. Formulating definite and complete answers to questions concerning the relationship between all surrogates widely used as metabolic control measures and patient-important endpoints seems to be an important challenge in contemporary diabetology. After new data from clinical studies currently underway becomes available and a complete systematic review of both new data and already published reports is performed, we may well be close to an answer.
References
- Montori VM, Permanyer-Miralda G, Ferreira-Gonzalez I, Busse JW, Pacheco-Huergo V, Bryant D, Alonso J, Akl EA, Domingo-Salvany A, Mills E, Wu P, Schünemann HJ, Jaeschke R, Guyatt GH. Validity of composite end points in clinical trials. BMJ 2005. 330:594-596. [DOD] [CrossRef]
- Psaty BM, Lumley T. Surrogate end points and FDA approval. A Tale of 2 lipid-altering drugs. JAMA 2008. 299:1474-1476. [DOD] [CrossRef]
- Guyatt G, Montori V, Devereaux PJ, Schünemann H, Bhandari M. Patients at the center: In our practice, and in our use of language. ACP J Club 2004. 140:A11-A12. [DOD]
- Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov 2003. 2:566-580. [DOD] [CrossRef]
- Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx 2004. 1:189-195. [DOD] [CrossRef]
- Prentice RL. Surrogate endpoints in clinical trials: definitions and operational criteria. Stat Med 1989. 8:431-440. [DOD] [CrossRef]
- Molenberghs G, Burzykowski T, Alonso A, Buyse M. A perspective on surrogate endpoints in controlled clinical trials. Stat Methods Med Res 2004. 13:177-206. [DOD]
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med 1998. 338:645-652. [DOD] [CrossRef]
- Tatti P, Pahor M, Byington RP, Di Mauro P, Guarisco R, Strollo G, Strollo F. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care 1998. 21:597-603. [DOD] [CrossRef]
- Psaty BM, Weiss NS, Furberg CD, Koepsell TD, Siscovick DS, Rosendaal FR, Smith NL, Heckbert SR, Kaplan RC, Lin D, Fleming TR, Wagner EH. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA 1999. 282:786-790. [DOD] [CrossRef]
- Buyse M, Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics 1998. 54:1014-1029. [DOD] [CrossRef]
- Friedman L. Interpreting results of trials using surrogate outcome measures. Evid Based Cardiovasc Med 1998. 2:61. [DOD] [CrossRef]
- Temple R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 1999. 282:790-795. [DOD]
- Wagner JA. Overview of biomarkers and surrogate endpoints in drug development. Dis Markers 2002. 18:41-46. [DOD]
- Deyton L. Improtance of surrogate markers in evaluation of antiviral therapy for HIV infection. JAMA 1996. 276:159-160. [DOD] [CrossRef]
- Riggs BL, Hodgson SF, O'Fallon WM, Chao EY, Wahner HW, Muhs JM, Cedel SL, Melton LJ 3rd. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990. 322:802-809. [DOD]
- Akiyama T, Pawitan Y, Greenberg H, Kuo CS, Reynolds-Haertle RA. Increased risk of death and cardiac arrest from encainide and flecainide in patients after non-Q-wave acute myocardial infarction in the Cardiac Arrhythmia Suppression Trial. CAST Investigators. Am J Cardiol 1991. 68:1551-1555. [DOD] [CrossRef]
- American Diabetes Association. Diabetic nephropathy position statement. Diabetes Care 2001. 24:S69-S72. [DOD] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes 2006. Diabetes Care 2006. 29:S4-S42. [DOD]
- Turner RC, Holman RR, Stratton IM, Cull CA, Matthews DR, Manley SE, et al. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998. 352:854-865. [DOD] [CrossRef]
- DCCT Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993. 329:977-986. [DOD] [CrossRef]
- Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O'Leary DH, Genuth S, Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003. 348:2294-2303. [DOD] [CrossRef]
- EDIC group. Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Diabetes 1999. 48:383-390. [DOD] [CrossRef]
- Kawasumi M, Tanaka Y, Uchino H, Shimizu T, Tamura Y, Sato F, Mita T, Watada H, Sakai K, Hirose T, Kawamori R. Strict glycemic control ameliorates the increase of carotid IMT in patients with type 2 diabetes. Endocr J 2006. 53:45-50. [DOD] [CrossRef]
- Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation 1993. 87(3 Suppl):II56-II65. [DOD]
- Burke GL, Evans GW, Riley WA, Sharrett AR, Howard G, Barnes RW, Rosamond W, Crow RS, Rautaharju PM, Heiss G. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 1995. 26:386-391. [DOD]
- Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997. 96:1432-1437. [DOD]
- American Diabetes Association. Postprandial Blood Glucose. Diabetes Care 2001. 24:775-778. [DOD] [CrossRef]
- Monnier L, Benichou M, Charra-Ebrard S, Boegner C, Colette C. An overview of the rationale for pharmacological strategies in type 2 diabetes: from the evidence to new perspectives. Diabetes Metab 2005. 31:101-109. [DOD] [CrossRef]
- Monnier L, Lapinski H, Colette C. Contribution of fasting and postprandial glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients. Diabetes Care 2003. 26:881-924. [DOD] [CrossRef]
- Woerle HJ, Neumann C, Zschau S, Tenner S, Irsigler A, Schirra J, Gerich JE, Göke B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007. 77:280-285. [DOD] [CrossRef]
- Ungar G, Freedman L, Shapiro S. Pharmacological studies of a new oral hypoglycemic drug. Proc Soc Exp Biol Med 1957. 95:190-192. [DOD]
- US Food and Drug Administration. FDA Approves New Diabetes Drug. Press release, December 30, 1994. Available online at http://www.fda.gov/bbs/topics/ANSWERS/ANS00627.html, retrieved on October 20, 2008. [DOD]
- Johansen K. Efficacy of metformin in the treatment of NIDDM. Meta-analysis. Diabetes Care 1999. 22:33-37. [DOD] [CrossRef]
- Saenz A, Fern-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005. 3:CD002966. [DOD]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G, ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006. 355(23):2427-2443. [DOD] [CrossRef]
- Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ, RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes - an interim analysis. N Engl J Med 2007. 357:28-38. [DOD] [CrossRef]
- Komajda M, Curtis P, Hanefeld M, Beck-Nielsen H, Pocock SJ, Zambanini A, Jones NP, Gomis R, Home PD, RECORD Study Group. Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: a randomized controlled trial (the RECORD study). Cardiovasc Diabetol 2008. 7:10. [DOD] [CrossRef]
- Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, Wiley C, Selvin E, Wilson R, Bass EB, Brancati FL. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007. 147:386-399. [DOD]
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008. 358(24):2560-2572. [DOD] [CrossRef]
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005. 2:CD003639. [DOD]
- Black C, Donnelly P, McIntyre L, Royle PL, Shepherd JP, Thomas S. Meglitinide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007. 2:CD004654. [DOD]
- Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004. 25:10-16. [DOD] [CrossRef]
- Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med 1997. 126:376-380. [DOD]
- van de Laar FA, Lucassen PL. No evidence for a reduction of myocardial infarctions by acarbose. Eur Heart J 2004. 25(13):1179. [DOD] [CrossRef]
- Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008. 358(24):2545-2559. [DOD] [CrossRef]
- Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004. 141:421-431. [DOD]
- Groeneveld Y, Petri H, Hermans J, Springer MP. Relationship between blood glucose level and mortality in type 2 diabetes mellitus: a systematic review. Diabet Med 1999. 16:2-13. [DOD] [CrossRef]
|