Review
| Rev Diabet Stud,
2010,
7(2):82-92 |
DOI 10.1900/RDS.2010.7.82 |
Generation of Insulin-Producing Cells From Pluripotent Stem Cells: From the Selection of Cell Sources to the Optimization of Protocols
Chee-Gee Liew
UCR Stem Cell Center, University of California, Riverside, CA 92521, USA
Manuscript submitted July 4, 2010; resubmitted August 2, 2010; accepted August 4, 2010.
Keywords: stem cell, induced pluripotent, embryonic, transcription factor, definite endoderm, blastocyst, endocrine pancreas, brachyury, goosecoid, gene expression
Abstract
The pancreas arises from Pdx1-expressing progenitors in developing foregut endoderm in early embryo. Expression of Ngn3 and NeuroD1 commits the cells to form endocrine pancreas, and to differentiate into subsets of cells that constitute islets of Langerhans. β-cells in the islets transcribe gene-encoding insulin, and subsequently process and secrete insulin, in response to circulating glucose. Dysfunction of β-cells has profound metabolic consequences leading to hyperglycemia and diabetes mellitus. β-cells are destroyed via autoimmune reaction in type 1 diabetes (T1D). Type 2 diabetes (T2D), characterized by impaired β-cell functions and reduced insulin sensitivity, accounts for 90% of all diabetic patients. Islet transplantation is a promising treatment for T1D. Pluripotent stem cells provide an unlimited cell source to generate new β-cells for patients with T1D. Furthermore, derivation of induced pluripotent stem cells (iPSCs) from patients captures "disease-in-a-dish" for autologous cell replacement therapy, disease modeling, and drug screening for both types of diabetes. This review highlights essential steps in pancreas development, and potential stem cell applications in cell regeneration therapy for diabetes mellitus.
Abbreviations: B-iPSC - blood cell-derived iPSC; BMP4 - bone morphogenetic protein 4; Bry - Brachyury; CACNA1A/C - calcium channel, voltage-dependent, P/Q type, alpha 1 A/C subunit; cAMP - cyclic adenosine monophosphate; c-Myc - v-myc myelocytomatosis viral oncogene homolog (avian, homo sapiens); CNS - central nervous system; Cre recombinase - type I topoisomerase (catalyzes site-specific recombination of DNA between loxP sites); CXCR4 - chemokine (C-X-C motif) receptor 4; DE - definitive endoderm; DiPSC - diabetes (T1D)-specific iPSC; EB - embryoid body; EGF - endothelial growth factor; eGFP - enhanced green fluorescent protein; ERK1/2 - extracellular-signal-regulated kinases 1 and 2; ESC - embryonic stem cell; EVX1 - even-skipped homeobox protein 1; ExEn - extraembryonic endoderm; FACS - fluorescence-activated cell sorting; F-iPSC - fibroblast-derived iPSC; FGF - fibroblast growth factor; Foxa1 - forkhead-box protein A1 (also termed Hnf3α); Foxa2 - forkhead-box protein A2 (also termed Hnf3β); GLUL - glutamate-ammonia ligase; Glut2 - glucose transporter 2 (also known as solute carrier family 2, member 2, SLC2A2); Gsc - goosecoid; Hes1 - hairy and enhancer of split 1 (transcription factor; controls differentiation and proliferation of neuronal, endocrine, and T lymphocyte progenitors during development); HESC - human embryonic stem cell; HGF1 - hepatocyte growth factor 1; HIF - hypoxia inducible factor; HNF - hepatocyte nuclear factor; IAPP - islet amyloid polypeptide; ICM - inner cell mass; IDE - inducer of definitive endoderm; IGF - insulin growth factor; ILV - indolactam V; iPSC - induced pluripotent stem cell; KAAD-Cyc - "3-Keto-N-(aminoethyl-aminocaproyl-dihydro-cinnamoyl)-cyclopamine; LGMN - legumain; LIF - leukemia inhibitory factor; MafA - v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (transcription factor necessary for beta-cell maturation); MafB - v-maf musculoaponeurotic fibrosarcoma oncogene homolog B (transcription factor important for alpha- and beta-cell development and mature alpha-cell function); MAPK - mitogen-activated protein kinase; Mixl1 - Mix-like 1 homeodomain protein (transcription factor; required for mesendoderm morphogenesis); MMTV - mouse mammary tumor virus; MODY - maturity onset diabetes of the young; NeuroD - neurogenic differentiation (also known as BETA2; transcription factor expressed in pancreatic cells); Ngn3 - neurogenin 3 (member of the bHLH family of transcription factors expressed in the nervous system); Nkx2.2 - homeobox protein and NK2 transcription factor related, locus 2 (involved in morphogenesis of the CNS); Nkx6.1 - homeobox protein required for β-cell development; ntESC - nuclear transfer ESC; Oct4 - octamer binding transcription factor 4; Pax4 - paired box gene 4 (transcription factor involved in fetal and pancreas development) ; Pdx1 - pancreatic and duodenal homeobox 1; PC1/3 - prohormone convertase 1/3; PE - parietal endoderm; PI3K - phosphoinositide 3-kinase; PKC - protein kinase C; pO2 - partial pressure of oxygen; PPY - pancreatic polypeptide; PrE - primitive endoderm; RA - retinoic acid; SHH - sonic hedgehog; Sox9 - sex determining region Y box 9 (transcription factor involved in skeleton development); Sox17 - sex determining region Y box 17 (endodermal transcription factor); STEMCCA - stem cell cassette; STZ - streptozotocin; T - symbol and gene name of brachyury (transcription factor expressed in the inner cell mass of the blastocyst); T1D - type 1 diabetes; T2D - type 1 diabetes; Tet-Off - tetracycline operator system; TGF-beta - transforming growth factor beta; VE - visceral endoderm; VEGF - vascular endothelial growth factor; VGCC - voltage-gated Ca2+ channel; WNT3a - wingless-type MMTV integration site family, member 3A
From stem cells in early embryo to pancreas
Definitive endoderm (DE) specification is the critical first step towards differentiation of pancreatic lineage from a pluripotent stem cell. Pluripotent stem cells are derived from the inner cell mass (ICM) of blastocysts, 3-5 days post-fertilization. In vivo, cells on the mural surface of ICM adjacent to the blastocoel cavity differentiate into primitive endoderm (PrE), which consists of precursor cells of extraembryonic endoderm (ExEn) characterized by Sox7 expression [1]. Subsequently, PrE differentiate into visceral endoderm (VE), and parietal endoderm (PE), forming the visceral and parietal yolk sac.
Gastrulation occurs shortly after implantation, during which cells that properly contribute to embryogenesis are called epiblast. Primitive streak mesendoderm, hallmarked by the expression of goosecoid, T (Brachyury), and Evx1, forms in a specific area of the epiblast along the posterior axis of the embryo. Epiblast cells move and spread through the streak, between ectoderm and VE cells, to form the mesoderm. Initiation of nodal signaling and expression of transcription factors Mixl1 and Sox17 induce DE formation from cells at the outer lining of the epiblast, which is initially occupied by VE cells. DE cells express Foxa1 and Foxa2, and later differentiate to form the epithelium of the primitive gut tube that gives rise to gastrointestinal and respiratory tracts, and organs of digestive tracts. The posterior gut endoderm develops into mid- and hindgut, that later give rise to the intestine. Pdx1-expressing cells are detected in both anterior and posterior foregut endoderm, which develop into ventral and dorsal pancreas respectively [2].
Fibroblast growth factor (FGF) signaling controls anterior-posterior axis formation during endoderm development in the embryo. In vivo, cardiac mesoderm-secreted FGF2 patterns the ventral foregut into liver and lung [3], whereas FGF2 secreted by notochord represses endodermal sonic hedgehog (SHH), and permits pancreas development [4]. Using human embryonic stem cells (HESCs) as an in vitro model, Ameri et al. showed that FGF2 is involved in foregut and hindgut specification, in a concentration-dependant manner [5]. In their study, low doses of FGF2 promoted a hepatic fate, while high doses induced anterior foregut and small intestinal cell development. Importantly, intermediate doses of FGF2 (64 ng/ml) promoted the formation of pancreatic cells, as proven by the immunoreactivity to Sox9, Nkx6.1, and Ngn3. FGF2-mediated Pdx1 induction is activated via FGF receptor signaling and ERK1/2 mitogen-activated protein kinase (MAPK) pathway. Nevertheless, in contrast to mouse development, FGF4 did not pattern HESC-derived DE cells towards posterior endoderm, but played a role in promoting cell survival during DE specification [6, 7]. Also, FGF10 is necessary for the proliferation of PDX1-expressing pancreatic epithelial progenitors, and pancreas branching morphogenesis [8].
Generating definitive endoderm and pancreatic endocrine cells from embryonic stem cells (ESCs)
Various approaches have been tested to induce DE from embryonic stem cells (ESCs). Using a Brachyury-eGFP (Bry-eGFP) mouse ESC reporter line, Kubo et al. defined a requirement for a member of the transforming growth factor β (TGF-β) family, activin A in the induction of this early lineage [9]. Bry-eGFP+ cells, derived from differentiating embryoid bodies (EBs), gave rise to both mesoderm and endoderm populations. Furthermore, Foxa2- and Sox17-expressing cells emerged from Bry-eGFP+ cells cultured in the presence of activin A in serum-free media. Yasunaga et al. further demonstrated that two distinct cell types can be separated from endoderm. They found that DE cells were derived from goosecoid- (Gsc) and Sox17-coexpressing cell population (Gsc+Sox17+), while VE cells were restricted in Gsc-Sox17+E-cadherin+ epithelial-like cells [10]. Also, microarray analysis revealed seven surface markers that are differentially expressed in DE and VE cells. One of these markers is cytokine receptor, CXCR4.
D'Armour demonstrated that in activin A-treated monolayer human ESC cultures, 80% of cells expressed Foxa2 and Sox17 [11]. During activin A treatment, undifferentiated cells were transitioned though mesendodermal pathway, accompanied by the transient expression of BRACHYURY, N-CADHERIN, WNT3a and FGF4. Also, Wnt3a directly induced BRACHYURY expression, and elicited a rapid and highly efficient cellular progression through primitive streak to DE [12]. Remarkably, the activin A-induced DE specification is efficient only when insulin/insulin growth factor (IGF) signaling is reduced, and when phosphoinositide 3-kinase (PI3K) signaling is suppressed [13]. These DE cells could be isolated and enriched by fluorescence-activated cell sorting (FACS) for CXCR4-expressing cells. Similar to Yasunaga's finding, CXCR4 expression in activin A-treated cultures also distinguished DE from PrE cells.
In the absence of serum, or in very low serum concentration, experiments with human and mouse ESCs provided evidence for effective DE induction by activin A. Furthermore, activin A induction is concentration-dependent. High concentration of activin A (50-100 ng/ml) is required for efficient DE induction [9, 14, 15]. The use of low activin A concentrations (5 -20 ng/ml) was sufficient to maintain undifferentiated feeder-free human ESCs in pluripotent state (Figure 1). Whereas, intermediate levels of activin A (20-50 ng/ml) provoked differentiation to mesoderm lineage [16]. However, in monolayer cultures, expression of Gsc and other anterior markers can be inhibited by bone morphogenetic protein-4 (BMP4), which may prolong Brachyury expression and lead to subsequent mesoderm development [17]. Intriguingly, simultaneous exposure of HESCs, differentiated to intermediate levels of BMP4, and activin A (50 ng/ml each) supported DE differentiation, evidenced by increased PDX1 transcript expression in embryoid bodies.
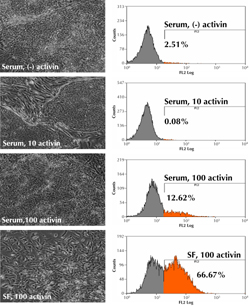 |
 |
Figure 1. Morphology and CXCR4 expression in human ESC cultures treated with different concentrations of activin A after 5 days. 10 ng/ml activin A maintains undifferentiated ESCs in the presence of 10% serum. 100ng/ml activin A induces epithelial-to-mesenchymal transition (EMT) only in serum-free (SF) cultures. EMT plays crucial roles in embryonic gastrulation, and gives rise to the mesoderm and endoderm [67]. FACS analysis shows the number of CXCR4-expressing cells in each culture condition. |
|
SHH inhibitor and FGF10 have been used to prime DE cells to form cells characteristic of primitive gut tube. At this stage, DE cell markers were downregulated, while HNF1B and HNF4A expressions increased. Retinoic acid (RA), a 'posteriorizing factor' and Notch inhibitor was included next to promote posterior foregut and endocrine cell formation, while suppressing exocrine program. The use of exedin-4, IGF1, and HGF1, at the later stage of differentiation, increased the formation of all pancreatic hormone-producing cell types. However, the inclusion of various signaling inhibitors always resulted in prominent cell death in cultures, hindering continuous culture and downstream analysis (our own observations).
Co-culturing of mouse ESCs with primary hepatocytes also induced the formation of homogenous monolayer of DE-like cells [18]. These DE cells could be coaxed into endocrine pancreas by plating on Matrigel using SHH inhibitor and RA. The resulting cells were pancreatic endocrine cells, demonstrated by upregulation of Pdx1 transcript and protein levels. These cells were co-cultured with cardiac microvascular endothelial cells and Notch inhibitor to prompt islet cell maturation. The outcome of this protocol is a large-scale generation of morphologically homogenous cultures that contain 60% cells co-expressing Pdx1 and C-peptide [18]. These findings have drawn two important conclusions: first, direct co-culture significantly increases Pdx1 and insulin production, compared to the use of conditioned medium or transwell indirect cultures. This suggests that cell-to-cell contact is a prerequisite for efficient pancreatic β-cell development. Second, direct co-culture efficiently enhances cell survival, while the Notch signaling pathway is suppressed in the presence of various antagonists.
Screening of small molecule libraries is fast becoming an increasingly popular tool to unravel the various pathways that control lineage commitment in stem cells. Small molecules are low molecular weight organic compounds that have high affinity to biopolymers, such as protein and nucleic acid. Several researcher groups have discovered small molecules that increase the production of pancreatic progenitors from mouse and human ESCs. In most studies, activin A was first used to specifically induce DE cell formation. For example, treatment of mouse ESCs with activin A and stauprimide increased the percentage of Sox17+N-cadherin+ DE cell population to 60%. Treatment with stauprimide alone did not result in DE induction [19]. Interestingly, expression of mesendoderm marker Brachyury was upregulated, suggesting that the formation of DE cells in vitro closely reproduced the in vivo process.
High content chemical screenings have also revealed new small molecules involved in inducing DE cells from ESCs. Borowiak et al. reported that IDE1 and IDE2 induced nearly 80% of ESCs to form SOX17-expressing DE cells, a higher efficiency than that achieved by activin A or Nodal [20, 21]. Studies with Pdx1-eGFP reporter line also confirmed that these DE cells were able to further differentiate into pancreatic progenitors. These chemically-induced DE cells were able to incorporate into the gut tube of mouse embryos in vivo, indicating full functional competency of the cells. However, it is unknown whether IDE1 and IDE2 mediate DE formation through mesendoderm induction. On the other hand, (-)-indolactam V (ILV) is also found to be a potent inducer of pancreatic progenitor formation from human ESCs. ILV activated protein kinase C (PKC) signaling, and specifically targeted at one stage of pancreatic development, by inducing Pdx1-expressing cells from gut tube endoderm [22]. PKC signaling is thus implicated in the induction and maintenance of Pdx1+ cells. The role of PKC agonists should further be exploited in generating pancreatic β-cells from DE and ESCs.
The protocol to generate islet cell types, especially β-cells from pancreatic endoderm, is less defined. In vivo, islet-like cell clusters, isolated from fetal pancreas obtained during second trimester, contain less than 10% β-cells. These insulin-producing β-cells are Ki67-positive and highly proliferative. Subsequently, they constitute 50% of cells found in adult islets [23, 24]. Human fetal β-cells are able to release insulin in response to secretagoues that elevate cAMP levels, but they secrete little or no insulin when challenged with glucose [25]. β-cells have also been derived from DE cells cultured in a differentiation cocktail consisting of FGF10, epidermal growth factor (EGF), VEGF, forskolin, HGF, and pancreatic polypeptide (PPY). The use of exedin 4, IGF1, and HGF resulted in insulin-producing cells that responded to β-cell secretagogues, but only minimally to glucose, indicating the immature status of the cells. However, Ngn3- and Nkx6.1-expressing pancreatic endoderm generated glucose-responsive endocrine and insulin-producing cells after transplantation into mice. Additionally, the use of FGF2/nicotinamide and transfer of differentiated cells to aggregate cultures was found to generate insulin-producing cells that responded to high glucose stimulation [26]. This finding suggests that in vivo maturation and reaggregation are necessary to generate glucose-responsive adult β-cells from human ESCs. Figure 2 summarizes specification, commitment, and DE formation of endocrine pancreas and β-cells from an undifferentiated stem cell population.
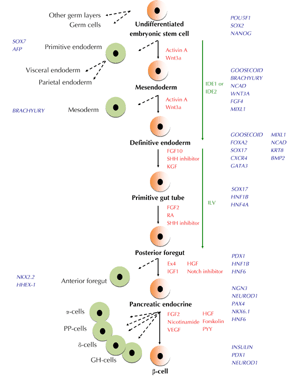 |
 |
Figure 2. Schematic representation of pancreatic endocrine β-cell formation from pluripotent stem cells. Hallmarks of transcription factors expressed at different stage are shown in blue, signaling pathway/molecules involved in differentiation are shown in red, and small molecules used are shown in green. It is currently unknown whether IDE1 and IDE2 induce definitive endoderm (DE) differentiation from bipotent mesendoderm cells. Dashed arrows indicate other cell types that are generated apart from cells differentiating towards the β-cell pathway. FGF: fibroblast growth factor. SHH: Sonic Hedgehog. KGF: keratinocyte growth factor. RA: retinoic acid. Ex: exedin. IGF: insulin growth factor. HGF: hepatocyte growth factor. VEGF: vascular endothelial growth factor. PYY: pancreatic polypeptide. GH-cells: ghrelin-producing cells. |
|
Genetic manipulation
Constitutive expression of pdx1 or pax4 in mouse ESCs promote differentiation of nestin-positive progenitors and insulin-producing cells. During in vitro differentiation, important changes in expression levels of pancreatic genes were detected in Pax4+ cells, and to a lesser extent, in Pdx1+ cells. IAPP, GLUT2, and Insulin mRNA were upregulated in Pax4+ cells at a later stage of differentiation, although ngn3 expression remained constant, suggesting that ngn3 is upstream of pax4 expression [27]. Pax4+ cells also contained an increased level of intracellular insulin. When cultivated in ‘Spinner’ bioreactors these cells undergo histotypic maturation to yield spheroid islet-like clusters grown in suspension. Electron microscopy detected insulin secretory granules, a characteristic of β-cells in adult islets [28]. Moreover, Pax4+ cells were glucose-responsive, and were able to normalize blood glucose levels in streptozotocin (STZ)-treated diabetic mice.
Overexpression of Foxa2 or Pdx1 has minimal effect in promoting pancreatic differentiation in human ESCs. Surprisingly, Foxa2 upregulated PAX6 expression, a neuroectodermal marker in EBs. Pdx1 overexpression transiently upregulated NGN3 and NKX2.2 transcript expression, but did not induce insulin production even in later stages of differentiation. Pdx1 is required for pancreas morphogenesis in early embryo, and is downregulated during endocrine specification. However, Pdx1 expression is upregulated, and is thereby maintained in mature β-cells for normal glucose homeostasis and regulation of insulin production. Thus, Pdx1 only exerts its effect in early pancreas development, but is not sufficient to induce the formation of pancreatic endocrine from human ESCs. Reporter lines created from stably transfected human ESCs revealed the appearance of Pdx1-eGFP+ cells in EBs, but insulin-eGFP+ cells could not be detected at any stage of differentiation.
PAX4 is a switch between β- and α-cells. Based on its onset of activation prior to β-cell specification in developing pancreas, we have tested the function of Pax4 in committing human ESCs to differentiate into β-cells. Constitutive expression of Pax4 in human ESCs substantially enhanced their propensity to form β-like cells, even without culture manipulation [29]. These cells upregulated INSULIN, PDX1, GLUT2, and Prohormone convertase 1/3 (PC1/3) transcripts, contained C-peptide, and responded to depolarizing concentration of KCl in a manner consistent with an action on voltage-gated Ca2+ channel (VGCC) gene expression. Pax4+ cells are also found to potentiate the expression of VGCC genes associated with the endocrine pancreas, such as CACNA1A and CACNA1C [30].
Transcriptional signaling during in vivo pancreas and islet development is a tightly controlled process. In most of the studies described above, transcription factors are overexpressed at a much earlier time. Nevertheless, precocious expression of these factors results in the formation of insulin-producing cells that retain expression of immature genes. This caveat can be circumvented by transiently and timely expressing genes of interest to promote formation of a desired cell type. For example, mouse EBs induced to express ngn3 were more sensitive to Notch-signaling inhibition. Therefore, they upregulated islet transcripts in differentiating cell population [31]. However, immunocytochemistry analysis revealed the predominant appearance of α- and somatostatin-expressing cells at the expense of other endocrine cells. On the other hand, conditional induction of Pdx1, using a Tet-Off inducible system in mouse EBs, resulted in upregulation of β-cell markers in a nestin-selection differentiation culture [32, 33]. Nevertheless, glucose responsiveness was not observed in these cells.
Cre-mediated induction of Sox17 in human ESCs gave rise to DE progenitors that upregulated HNF4a, CXCR4, and FOXA2 transcripts [34]. These Sox17-induced progenitors can be passaged and stably maintained in culture. As expected, these Sox17+ cells are multipotent. They can be directed to differentiate into hepatic and pancreatic lineages. In vivo studies revealed that teratoma formed by injection of Sox17+ cells contained a mixture of mesodermal and endodermal structures, with no evidence of ectoderm derivatives. They also gave rise to Pdx1 and insulin-producing pancreatic endocrine cells, when cultured in condition that favors pancreatic endocrine differentiation. Notably, activin A is not required during differentiation, possibly due to sustained endogenous Sox17 expression in the cells.
In future, a more deliberate genetic manipulation approach should be applied to induce pancreatic endocrine cells to undergo stepwise differentiation into more mature stages. The use of an in vitro inducible gene expression system to switch genes 'on and off' will faithfully recapitulate signaling cascades that regulate gene expression in vivo. Bernardo et al. have recently demonstrated that biphasic Pdx1 induction in mouse, and human, ESCs closely mimics pancreatic β-cell development [35]. Early and late Pdx1 induction, with intermediate withdrawal during in vitro differentiation, resulted in the highest upregulation of all islet endocrine transcripts expressed in cells differentiated from DE precursors. Despite this exciting finding, these cells expressed MAFB instead of MAFA, unlike adult β-cells. This feature is characteristic of partially differentiated pancreatic endocrine cell types [36]. Ultimately, timely expression of other critical transcription factors such as Pax4 and Nkx6.1 may be able to promote full maturation of these cells.
Effect of oxygen and glucose on embryonic pancreas differentiation
Earliest stages of human embryogenesis occur in a hypoxic, or even anoxic, environment [37], a process implicated as critical for stem cells expansion. Recent evidence has identified a broader spectrum of stem cells, including ESCs, hematopoietic, mesenchymal, and neural stem cells influenced by differing oxygen concentration in culture [38]. At low oxygen tension (pO2), cells exerted physiological response to ensure sufficient levels for oxygen-dependent processes. Hypoxia-inducible factors (HIFs) were activated upon the exposure of cells to hypoxic condition, and regulated the expression of genes related with proliferation, differentiation, glycolysis, and apoptosis [39]. Hypoxia decreased the proliferation rate of embryonic pancreata [40]. In doing so, hypoxia induced HIF1α and Hes1 expression in embryonic pancreata, which in turn repressed Ngn3 expression, and prevented endocrine cell differentiation. On the other hand, there was a dramatic increase in the number of various islet cells when the embryonic pancreata were cultured in 80% O2. Thus, hypoxia can be induced in DE cells to expand pancreatic progenitors. Whereas, higher oxygen concentrations can be used at the later stage of differentiation to obtain β-cells.
In the adult pancreas, glucose is the major insulin secretagogue, and plays a role in β-cell proliferation [41]. Increased glucose concentration (10 mM) in adult islets leads to Pdx1 phosphorylation and nuclear translocation, where it binds to and transactivate the Insulin promoter [42, 43]. In embryonic pancreas primordium, endocrine cell development is activated in the presence of glucose in a dose-dependent manner (up to 10 mM), while acinar cell formation is glucose-independent [43]. In this context, glucose controls embryonic β-cell differentiation by regulating NeuroD expression, rather than stimulating expansion of pancreas progenitors. Also, hyperglycemia induced residual β-cell proliferation, and thus increased β-cell mass in an autoimmune diabetes model [44]. Similarly, glucose and FGF2 acted together to induce NEUROD and INSULIN expression in suspension cultured EBs from human ESCs, but it did not exert an effect on inducing earlier endocrine markers such as NGN3 [45]. Taken together, regulation of oxygen tension, and the use of glucose at different stages of differentiation, should be explored further to derive pancreatic endocrine cells and β-cells from mouse and human ESCs.
"Reprogramming" in existing pancreas cells
Earlier studies have shown that transdifferentiation of cells to other lineages is possible. For example, ectopic expression of pdx1 is sufficient to induce expression of Insulin in mouse liver cells. Although transformed cells did not resemble functional β-cells in morphology, hyperglycemia in the host mice was reversed after in vivo transplantation [46]. Others have demonstrated that fully differentiated exocrine cells activate endocrine programs, when dissociated and cultured in vitro in the presence of leukemia inhibitory factor (LIF), EGF, or nicotinamide [47-49].
An emerging strategy for β-cell generation is inspired by the recent success of genetic manipulation and nuclear reprogramming in somatic cell types. By transiently expressing Ngn3, Pdx1, and MafA transcription factors with adenoviruses, 20% of mature pancreatic exocrine cells in adult mice were directly converted to β-cells [50]. Although these induced β-cells did not self-cluster, they remodeled local vasculature, secreted insulin, and ameliorated hyperglycemia. Thus, future work should focus on promoting aggregation of these transformed β-cells, which may fully restore glucose responsiveness in the mice. As pancreatic exocrine cells are the most abundant cell type in the pancreas, it is noteworthy that they are a potential surrogate source to generate β-cells for autologous cell replacement therapy in T1D patients.
Identification of novel transcription factors or cell surface markers may provide more information about pancreas development, and may significantly improve β-cell regeneration from stem cells. Partial pancreatectomy has been used as a model for pancreatic regeneration, as damaged pancreases secrete factors that stimulate differentiation or proliferation. Using a technique called suppression subtraction hybridization, which makes use of RNA extracted from injured and normal pancreas, Choi et al. have isolated twelve genes that were upregulated following partial pancreatectomy [51]. Several of these genes, namely GLUL, LGMN, and REG31 increased islet transcription factor expressions in Panc-1 and PC12 cell lines. Nevertheless, overexpression of these novel genes did not result in an increase in insulin-positive cells from mouse and human ESCs in vitro. However, overexpression of GLUL, LGMN, and REG31 may exert positive effects in generating β-cells from DE or pancreas endocrine cells. Thus, further studies on the roles of these novel genes are warranted.
Induced pluripotent stem cells and "disease-in-a-dish"
Derivation of induced pluripotent stem cells (iPSCs) has opened up opportunities to generate β-cells from individuals with diabetes mellitus. Human and mouse somatic cells can be reprogrammed to pluripotency by the introduction of two sets of transcription factors, either Oct4, Sox2, Klf4, and cMyc, or Oct4, Sox2, Nanog, and Lin28 (at 0.01% reprogramming efficiency) [52-54]. The inclusion of cMyc markedly enhanced iPSC generation, but also increased tumorigenicity in the resulting chimeras and progeny mice [55]. Recently, another Myc member, L-Myc was shown to specifically promote formation of fully reprogrammed iPSC colonies in human, and to promote germline transmission, but not tumor formation in the iPSC-derived mice [56]. Therefore, the use of L-Myc should be considered for future clinical applications.
Despite the remarkable similarity between iPSCs and ESCs, there is emerging evidence for subtle differences between these cells types. While some iPSC clones contribute poorly to chimeras [57], different iPSC lines derived from different somatic cell types have distinctive in vitro and in vivo (teratoma) differentiation properties [58]. Several groups have recently demonstrated that iPSCs indeed retain epigenetic memory of their somatic cells of origin. To test this hypothesis, Kim et al. differentiated fibroblast-derived iPSCs (F-iPSCs) and blood cell-derived iPSCs (B-iPSCs) into hematopoietic colonies and osteoblasts. F-iPSCs and B-iPSCs exhibited higher potential to form hematopoietic colonies and osteoblasts than mESCs and ESCs derived by nuclear transfer (ntESCs), respectively. This observation was surprising since their efficiency to differentiate into these two lineages is lower than that of mESCs and ntESCs [59, 60]. However, treatment with chromatin-modifying drugs, continuous passaging, differentiation, and serial reprogramming, largely attenuate the marked differences in differentiation propensity [60]. Hence, though iPSCs can be genetically identical, they are transcriptionally distinguishable based on their origin cell types. This is an important factor that can affect many differentiation protocols for disease modeling and patient-specific cell replacement therapies.
Novel techniques have been introduced over the years to generate transgene-free iPSCs suitable for use in clinical protocols. MicroRNAs have been shown to improve the reprogramming process, and to reduce heterogeneity in iPSC colonies [61]. Another advantage is that the use of recombinant proteins eliminated the risk of transgene integration in human iPSCs, even though the current success rate is 10-fold lower than that of the viral method [62]. Recent work, involving the use of a single excisable lentiviral "stem cell cassette" (STEMCCA), has produced mouse iPSCs that contributed to embryos with normal developmental morphology, and live chimeric mice [63]. This finding indicates that transgene-free iPSCs have improved the differentiation potential, and should be taken into account for the clinical application of iPSCs. Notably, multiple transgene-free disease-specific human iPSC lines have been derived, using excisable STEMCCA from human patients with cystic fibrosis, sickle cell disease, and other lung-related diseases [64].
Human iPSC lines from patients with other genetic diseases, including type 1 diabetes (T1D), have been derived [65]. Although not necessarily free of viral vectors, T1D-specific iPSCs (DiPSCs) provide a tool to study diabetes mellitus by modeling 'disease-in-a-dish'. DiPSCs were able to develop into cell types of three germ layers, following in vitro differentiation [66]. These DiPSCs responded to TGF-β/Nodal signaling, and were able to differentiate along the endodermal and pancreatic lineages, following treatment of activin A, FGF10, KAAD-Cyc, and RA. Hence, these cells can be used to generate new β-cells for the original donors. Nevertheless, one would expect disease-specific iPSCs to closely mimic the phenotype of parental cells. Thus, long-term potential of DiPSCs to differentiate into pancreatic β-cells should be elucidated to investigate possible T1D onset later.
It is also mentionable that iPSCs provide a tool for toxicity testing and drug screening. Such applications may be useful in screening for potential chemicals, or environmental toxins, that contribute to the development of adult-onset type 2 diabetes (T2D), and maturity onset diabetes of the young (MODY). While stem cell is an obvious cell source for generating pancreatic β-cells to replace damaged cells for T1D patients, T2D is proven to be more difficult to treat, due to insulin resistance and relative insulin deficiency. Nonetheless, disease-specific human iPSCs provide a good model for investigating the effectiveness and potency of new drugs in treating T2D and MODY in preclinical applications.
Future prospects for stem cells in treating diabetes mellitus
Stem cells have shown promise to revolutionize the treatment of many degenerative diseases, including type 1 diabetes. Many studies provide evidence that ESC- and iPSC-derived β-cells in vitro are likely to mimic fetal β-cells, and are not fully functional. These cells could potentially be used to screen for new drugs to treat insulin secretory defects in T2D patients. Although patient-specific iPSCs are a potential source for autologous β-cell replacement therapy, there are transcriptional differences between ESC and iPSCs. Hence, further studies are required to ensure consistency and safety of pluripotent stem cells before they can be used in differentiation assays and future cell regenerative therapy.
Disclosures (conflict of interests statement): The author reports no conflict of interests.
Acknowledgments:
The author would like to thank Linh Vuong for critical reading of the manuscript.
References
- Shimosato D, Shiki M, Niwa H. Extra-embryonic endoderm cells derived from ES cells induced by GATA Factors acquire the character of XEN cells. BMC Dev Biol 2007. 7:80. [DOD] [CrossRef]
- Jonsson J, Ahlgren U, Edlund T, Edlund H. IPF1, a homeodomain protein with a dual function in pancreas development. Int J Dev Biol 1995. 39:789-798. [DOD]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development 2005. 132(1):35-47. [DOD] [CrossRef]
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev 1998. 12:1705-1713. [DOD] [CrossRef]
- Ameri J, Stahlberg A, Pedersen J, Johansson JK, Johannesson MM, Artner I, Semb H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells 2009. 28:45-56. [DOD]
- Elghazi L, Cras-Meneur C, Czernichow P, Scharfmann R. Role for FGFR2IIIb-mediated signals in controlling pancreatic endocrine progenitor cell proliferation. Proc Natl Acad Sci USA 2002. 99(6):3884-3889. [DOD] [CrossRef]
- Johannesson M, Stahlberg A, Ameri J, Sand FW, Norrman K, Semb H. FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner. PLoS One 2009. 4:e4794. [DOD] [CrossRef]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 2001. 128:5109-5117. [DOD]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development 2004. 131:1651-1662. [DOD] [CrossRef]
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol 2005. 23:1542-1550. [DOD] [CrossRef]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotech 2005. 23:1534-1541. [DOD] [CrossRef]
- Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA 2008. 105:12301-12306. [DOD] [CrossRef]
- McLean AB, D'Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE, Dalton S. Activin A efficiently specifies definitive endoderm from human embryonic stem cells only when Phosphatidylinositol 3-Kinase signaling is suppressed. Stem Cells 2007. 25:29-38. [DOD] [CrossRef]
- Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia 2007. 50:1228-1238. [DOD] [CrossRef]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development 2004. 131:1651-1662. [DOD] [CrossRef]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 2005. 118:4495-4509. [DOD] [CrossRef]
- Hansson M, Olesen DR, Peterslund JM, Engberg N, Kahn M, Winzi M, Klein T, Maddox-Hyttel P, Serup P. A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells. Dev Biol 2009. 330:286-304. [DOD] [CrossRef]
- Banerjee I, Sharma N, Yarmush M. Impact of co-culture on pancreatic differentiation of embryonic stem cells. J Tissue Eng Regen Med 2010. In press. [DOD]
- Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY, Wu X, Schultz PG. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell 2009. 4:416-426. [DOD] [CrossRef]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 2009. 4:348-358. [DOD] [CrossRef]
- Borowiak M. The new generation of beta-cells: replication, stem cell differentiation, and the role of small molecules. Rev Diabet Sud 2010. 7(2):93-104. This issue. [DOD] [CrossRef]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 2009. 5:258-265. [DOD] [CrossRef]
- Tu J, Tuch BE. Expression of Glucokinase in Glucose-Unresponsive Human Fetal Pancreatic Islet-Like Cell Clusters. J Clin Endocrinol Metab 1997. 82:943-948. [DOD] [CrossRef]
- Scharfmann R, Xiao X, Heimberg H, Mallet J, Ravassard P. Beta cells within single human islets originate from multiple progenitors. PLoS One 2008. 3:e3559. [DOD] [CrossRef]
- Hoffman L, Mandel T, Carter W, Koulmanda M, Martin F. Insulin secretion by fetal human pancreas in organ culture. Diabetologica 1982. 23:426-430. [DOD]
- Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M, Li D, Deng H. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res 2007. 17:333-344. [DOD] [CrossRef]
- Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA 2003. 100(3):998-1003. [DOD] [CrossRef]
- Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiol Rev 1981. 61:914-973. [DOD]
- Liew CG, Shah NN, Briston SJ, Shepherd RM, Khoo CP, Dunne MJ, Moore HD, Cosgrove KE, Andrews PW. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS ONE 2008. 3:e1783. [DOD] [CrossRef]
- Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev 2006. 27:621-676. [DOD] [CrossRef]
- Treff NR, Vincent RK, Budde ML, Browning VL, Magliocca JF, Kapur V, Odorico JS. Differentiation of embryonic stem cells conditionally expressing neurogenin 3. Stem Cells 2006. 24:2529-2537. [DOD] [CrossRef]
- Miyazaki S, Yamato E, Miyazaki JI. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes 2004. 53:1030-1037. [DOD] [CrossRef]
- Liew CG, Andrews PW. Stem cell therapy to treat diabetes mellitus. Rev Diabet Stud 2008. 5(4):203-219. [DOD] [CrossRef]
- Seguin CA, Draper JS, Nagy A, Rossant J. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell 2008. 3:182-195. [DOD] [CrossRef]
- Bernardo AS, Cho CH, Mason S, Docherty HM, Pedersen RA, Vallier L, Docherty K. Biphasic induction of Pdx1 in mouse and human embryonic stem cells can mimic development of pancreatic beta-cells. Stem Cells 2009. 27:341-351. [DOD] [CrossRef]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol 2006. 293:526-539. [DOD] [CrossRef]
- Sonia PL, Ana C, Ana AN, Magdalena ML, Carmen EL, Carolina G, Sonia T, Dario M, David B, David M, et al. Hypoxia promotes efficient differentiation of human embryonic stem cells to functional endothelium. Stem Cells 2010. 28:407-418. [DOD]
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 2010. 7:150-161. [DOD] [CrossRef]
- Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2010. 139(1):85-97. [DOD] [CrossRef]
- Heinis M, Simon MT, Ilc K, Mazure NM, Pouyssegur J, Scharfmann R, Duvillie B. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 2010. 59:662-669. [DOD] [CrossRef]
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 1989. 38:49-53. [DOD] [CrossRef]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994. 371:606-609. [DOD] [CrossRef]
- Guillemain G, Filhoulaud G, Da Silva-Xavier G, Rutter GA, Scharfmann R. Glucose is necessary for embryonic pancreatic endocrine cell differentiation. J Biol Chem 2007. 282:15228-15237. [DOD] [CrossRef]
- Pechhold K, Koczwara K, Zhu X, Harrison VS, Walker G, Lee J, Harlan DM. Blood glucose levels regulate pancreatic beta-cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice. PLoS One 2009. 4:e4827. [DOD] [CrossRef]
- Khoo ML, McQuade LR, Smith MS, Lees JG, Sidhu KS, Tuch BE. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: effect of glucose and basic fibroblast growth factor. Biol Reprod 2005. 73:1147-1156. [DOD] [CrossRef]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatice and duodenal homeobox 1 gene induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 2000. 6(5):568-572. [DOD] [CrossRef]
- Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 2005. 48:49-57. [DOD] [CrossRef]
- Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA 2005. 102:15116-15121. [DOD] [CrossRef]
- Okuno M, Minami K, Okumachi A, Miyawaki K, Yokoi N, Toyokuni S, Seino S. Generation of insulin-secreting cells from pancreatic acinar cells of animal models of type 1 diabetes. Am J Physiol Endocrinol Metab 2007. 292:E158-E165. [DOD] [CrossRef]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008. 455:627-632. [DOD] [CrossRef]
- Choi JH, Lee MY, Kim Y, Shim JY, Han SM, Lee KA, Choi YK, Jeon HM, Baek KH. Isolation of genes involved in pancreas regeneration by subtractive hybridization. Biol Chem 2010. In press. [DOD]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006. 126:663-676. [DOD] [CrossRef]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007. 131:861-872. [DOD] [CrossRef]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007. 318:1917-1920. [DOD] [CrossRef]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotech 2008. 26:101-106. [DOD] [CrossRef]
- Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci USA 2010. 107:14152-14157. [DOD] [CrossRef]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 2010. 465:175-181. [DOD] [CrossRef]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 2009. 27:743-745. [DOD] [CrossRef]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010. In press. [DOD]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 2010. 28:848-855. [DOD] [CrossRef]
- Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol 2009. 27:459-461. [DOD] [CrossRef]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009. 4:472-476. [DOD] [CrossRef]
- Sommer CA, Sommer AG, Longmire TA, Christodoulou C, Thomas DD, Gostissa M, Alt FW, Murphy GJ, Kotton DN, Mostoslavsky G. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells 2010. 28:64-74. [DOD]
- Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, et al. Generation of transgene-free lung disease-specific human iPS cells using a single excisable lentiviral stem cell cassette. Stem Cells 2010. In press. [DOD]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell 2008. 134:877-886. [DOD] [CrossRef]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA 2009. 106:15768-15773. [DOD] [CrossRef]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009. 119:1420-1428. [DOD] [CrossRef]
This article has been cited by other articles:
|
Diabetes mellitus and cellular replacement therapy: Expected clinical potential and perspectives
Berezin AE
World J Diabetes 2014. 5(6):777-786
|
|

|
MiRNA-375 promotes beta pancreatic differentiation in human induced pluripotent stem (hiPS) cells
Lahmy R, Soleimani M, Sanati MH, Behmanesh M, Kouhkan F, Mobarra N
Mol Biol Rep 2014. 41(4):2055-2066
|
|

|
Generation of insulin-producing cells from rat mesenchymal stem cells using an aminopyrrole derivative XW4.4
Ouyang J, Huang W, Yu W, Xiong W, Mula RV, Zou H, Yu Y
Chem Biol Interact 2014. 208:1-7
|
|

|
Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors
Bhonde RR, Sheshadri P, Sharma S, Kumar A
Int J Biochem Cell Biol 2014. 46:90-102
|
|

|
Influences of the diabetes surgery on pancreatic β-cells mass
Martinez-Moreno JM, Garciacaballero M
Nutr Hosp 2013. 28(Suppl 2):88-94
|
|

|
Pancreatic islet differentiation of human embryonic stem cells by microRNA overexpression
Lahmy R, Soleimani M, Sanati MH, Behmanesh M, Kouhkan F, Mobarra N
J Tissue Eng Regen Med 2013. In press
|
|

|
MicroRNAs as pharmacological targets in diabetes
Mao Y, Mohan R, Zhang S, Tang X
Pharmacol Res 2013. 75:37-47
|
|
|
Differentiation of the definitive endoderm from Wharton's Jelly Mesenchymal Stem Cells (WJMSC)
Allahbakhshi E, Bijan Nejad D, Shariati M, Tabendeh MR, Nezhad Dehbashi F, Hashemitabar M
J Biol Res (Thessaloniki) 2013. 20:217-227
|
|
|
The clinical and basic research of islet transplantation: A literature data analysis based on the Science Citation Index database
Cao XK, Liu SQ, Li R, Liu BL
Chin J Tissue Engin Res 2012. 16(18):3365-3374
|
|

|
Oligodeoxynucleotide IMT504: lack of effect on immune parameters during islet regeneration in single dose streptozotocin-induced diabetes
Bianchi MS, Calvo V, Chasseing NA, Lago N, Libertun C, Montaner AD, Lux-Lantos VA
Diabetes Metab Res Rev 2012. 28(2):156-163
|
|
|
Effect of transcription factors on differentiation into insulin-producing cells from stem cells
Chen T, Qi H, Li FR
Chin Bull Life Sci 2012. 24(2):145-149
|
|

|
Position Statement of the Chinese Diabetes Society regarding stem cell therapy for diabetes
Zhu D, Chen L, Hong T
J Diabetes 2012. 4(1):18-21
|
|

|
Stem cell-based immunomodulation in type 1 diabetes: beyond the regenerative approach
Longoni B, Mosca F
Curr Pharm Des 2011. 17(29):3229-3242
|
|
|
Stem cell therapy in diabetes mellitus: current trends
Sarath R, Rani VL
Int J Biomed Res 2011. 2(12):608-617
|
|
|
Pluripotent stem cells
Müller T, Blasczyk R
Tumor Diagn Ther 2011. 32(6):338-344
|
|
|