Original Data
| Rev Diabet Stud,
2011,
8(2):259-267 |
DOI 10.1900/RDS.2011.8.259 |
Results of Pancreas Transplantation Alone with Special Attention to Native Kidney Function and Proteinuria in Type 1 Diabetes Patients
Ugo Boggi1, Fabio Vistoli1, Gabriella Amorese1, Rosa Giannarelli2, Alberto Coppelli2, Rita Mariotti3, Lorenzo Rondinini3, Massimiliamo Barsotti4, Alberto Piaggesi2, Anna Tedeschi2, Stefano Signori1, Nelide De Lio1, Margherita Occhipinti2, Emanuela Mangione2, Diego Cantarovich4, Stefano Del Prato2, Franco Mosca5, Piero Marchetti2,6
1Division of General and Transplant Surgery in Uremic and Diabetic Patients, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy
2Department of Endocrinology and Metabolism, University of Pisa, Pisa, Italy
3Division of Cardiology, Cardiac and Thoracic Department, University of Pisa, Pisa, Italy
4Department of Nephrology, Transplantation and Dialysis 1, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
5Department of Oncology, Transplants and Advanced Technologies in Medicine, University of Pisa, Pisa, Italy
6Unit of Endocrinology and Metabolism of Transplantation, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
Address correspondence to: Ugo Boggi, e-mail: uboggi@patchir.med.unipi.it
Manuscript submitted May 26, 2011; resubmitted July 3, 2011; accepted July 29, 2011.
Keywords: diabetes, pancreas transplantation, retinopathy, diabetic nephropathy, diabetic neuropathy, glomerular filtration rate, proteinuria
Abstract
We report on our single-center experience with pancreas transplantation alone (PTA) in 71 patients with type 1 diabetes, and a 4-year follow-up. Portal insulin delivery was used in 73.2% of cases and enteric drainage of exocrine secretion in 100%. Immunosuppression consisted of basiliximab (76%), or thymoglobulin (24%), followed by mycophenolate mofetil, tacrolimus, and low-dose steroids. Actuarial patient and pancreas survival at 4 years were 98.4% and 76.7%, respectively. Relaparatomy was needed in 18.3% of patients. Restored endogenous insulin secretion resulted in sustained normalization of fasting plasma glucose levels and HbA1c concentration in all technically successful transplantations. Protenuria (24-hour) improved significantly after PTA. Renal function declined only in recipients with pretransplant glomerular filtration rate (GFR) greater than 90 ml/min, possibly as a result of correction of hyperfiltration following normalization of glucose metabolism. Further improvements were recorded in several cardiovascular risk factors, retinopathy, and neuropathy. We conclude that PTA was an effective and reasonably safe procedure in this single-center experience.
Abbreviations: ACE - angiotensin-converting enzyme; BMI - body mass index; CMV - cytomegalovirus; E/A velocity - ratio between early (E) and late (atrial - A) ventricular filling velocity; EC-MPA - enteric-coated mycophenolic acid; GFR - glomerular filtration rate; HbA1c - glycated hemoglobin; HDL - high-density lipoprotein; HLA - human leukocyte antigen; IU - international unit; LDL - low-density lipoprotein; MMF - mycophenolate mophetil; MNSI - Michigan Neuropathy Screening Instrument; PAK - pancreas after kidney transplantation; PANCREAS - Pancreas Allotransplantation for Diabetic Nephropathy and Mild Chronic REnal fAilure Stage Study; PTA - pancreas transplantation alone; RR - relative risk; SD - standard deviation; SPK - simultaneous pancreas and kidney transplantation ; T1D - type 1 diabetes; Tx - transplantation; UNOS - United Network of Organ Sharing
Introduction
Pancreas transplantation is a clinical option in the treatment of patients with type 1 diabetes (T1D) [1-3]. This procedure may be considered as a group of three separate clinical entities: simultaneous pancreas and kidney transplantation (SPK), pancreas after kidney (PAK), and pancreas transplant alone (PTA) [1-3]. It has been shown that SPK, by inducing insulin independence and replacing native renal function, has beneficial effects on diabetes complications and prolongs life expectancy [1-10].
The usefulness of pancreas transplantation alone (PTA) in T1D patients without advanced nephropathy (GFR ≥ 50 ml/min) is debated [1, 3, 5-8]. It is generally accepted that patients are eligible for a PTA if they have:
1. a history of frequent, acute, and severe metabolic complications (hypoglycemia, hyperglycemia, ketoacidosis) requiring medical intervention, or
2. severe clinical and emotional problems with exogenous insulin therapy that are incapacitating, or
3. consistent failure of insulin-based management to prevent acute complications [11].
PTA may also be considered for T1D patients who have a high risk of secondary diabetic complications (nephropathy, retinopathy, neuropathy), as proposed by a few authors and scientific diabetes societies [1, 3, 12]. Recent studies reported that after PTA the 5-year patient survival is 90% [13], and that pancreas graft half-life is 9 years [6]. Sustained normoglycemia improves several microvascular diabetic complications [1, 3, 10, 14, 15]. Although, it might not prolong life expectancy compared with patients on the waiting list [5-8]. Furthermore, immunosuppressant nephrotoxicity is expected to cause, or expedite, the progression of diabetic nephropathy towards end-stage renal failure [1, 3, 16, 17].
With all this in mind, we conducted an evaluation of our results following PTA in seventy-one T1D patients. Safety and efficacy were monitored throughout follow-up, with special attention to proteinuria and native kidney function.
Patients and methods
The aim of this study was to define safety and efficacy of PTA in T1D patients receiving a PTA at a single institution. Safety was defined as patient survival, lack of major adverse events, and preservation of renal function. Efficacy was defined as transplant ability to induce and maintain insulin independence, and evidence of a positive effect on the course of microvascular diabetic complications, with special attention to diabetic nephropathy.
Patients' characteristics
Data from 71 PTA performed between December 2000 and March 2007 were reviewed and analyzed. The study was performed with approval by the Ethics Committee of the University of Pisa. At the time of transplantation, patients showed the following characteristics:
- Age: 38.4 ± 8.5 years.
- Gender: 37 males and 34 females.
- Body mass index (BMI): 23.5 ± 3.0 kg/m2.
- Duration of diabetes: 23.7 ± 9.9 years.
- Daily insulin requirement: 44 ± 14 IU.
Forty patients (56%) received antihypertensive therapy (angiotensin-converting enzyme inhibitors, calcium channel blockers, beta-blockers, or a combination thereof), and eleven of them (15%) received lipid-lowering agents (mainly statins). PTA was indicated in 19 patients (27%) because of hyperlabile diabetes, defined as poor metabolic control with frequent episodes of unawareness hypoglycemia, despite intensive insulin regimens. In the remaining patients, poor metabolic control was accompanied by varying degrees of microvascular diabetic complications. According to previous studies [1-3], an estimated GFR of ≥ 50 ml/min was required to become eligible for PTA. No per-protocol pancreas or renal biopsies were performed.
Pancreas donors had the following characteristics:
- Age: 26.6 yrs (range 5 to 55).
- Gender: 53 males and 18 females.
- BMI: 23.0 ± 3.2 kg/m2.
- Cumulative HLA-A and HLA-B mismatch was 2.8 (range 1-4).
- Mean pancreas cold ischemia time was 11 hours and 36 minutes (range 8 to 18 hours).
Transplantation procedures
A detailed description of the surgical techniques employed at our institution for pancreas transplantation was reported previously [18, 19]. Briefly, all grafts were placed in the space behind the ascending colon and its mesentery. Enteric exocrine drainage was used in all recipients, while venous effluent was created either in the portal system (73.2%), or in the systemic circulation (26.8%). All patients received an induction treatment, consisting of basiliximab (20 mg) (Simulect, Novartis, Basel, Switzerland) in 54 recipients (76%), or antithymocyte globulin (1 mg/kg/day) (Thymoglobulin, Genzyme Corporation, Cambridge, MA) in the remaining 17 recipients (24%). The first dose of either antibody was administered before graft reperfusion. Thymoglobulin was given for 7 consecutive days, with the daily dose held if the total leukocyte count was <2,500/mm3, or if the lymphocyte count was <100/mm3. The same maintenance therapy, including tacrolimus (Prograf, Astellas Pharma, Tokio, Japan), mycophenolate mophetil (MMF, CellCept, Roche, Basel, Switzerland), or mycophenolic acid (EC-MPA, Novartis, Basel, Switzerland), together with steroids, were given to all recipients. The dose of tacrolimus was adjusted to maintain blood through levels of 10-15 ng/ml during the first month, and of 8-12 ng/ml thereafter. MMF and EC-MPA were given at the highest tolerated dose (initial dose of 2 g/day of MMF and 1440 mg/day of EC-MPA), mainly based on hematologic toxicity and gastrointestinal side effects.
At the last follow-up control, tacrolimus through levels were 8.8 ± 1.8 ng/ml, whereas MMF and EC-MPA doses were 1.3 ± 0.4 g/day and 0.9 ± 0.2 g/day, respectively. Steroids were tapered to 5 mg/day at 3 months post-transplantation. Finally, all patients received antimicrobial, antiviral, and antithrombotic prophylaxis as previously detailed [18, 19].
Follow-up assessment
Upon discharge, patients were followed up monthly up to six months post-transplantation, every three months up to 1 year, and every 6 months thereafter, unless otherwise necessary. The pancreas graft was considered functionally competent as long as fasting blood glucose, random blood glucose level, and glycated hemoglobin concentration (HbA1c) were within the normal range without any pharmacological antidiabetic therapy.
For the purpose of the present study, the following parameters were assessed before and 1, 2, 3, and 4 years post-transplantation:
- body weight,
- blood pressure (measured three times with a sphygmomanometer after sitting position for at least 10 minutes; the mean of the last two measurements was recorded),
- fasting plasma glucose,
- HbA1c,
- fasting C-peptide,
- fasting total cholesterol and triglycerides,
- HDL cholesterol and LDL cholesterol.
Complete cardiac evaluation, including Doppler echocardiography (Sonos 5500 echograph; Agilent Technologies, Andover, MA), was performed, with geometric, systolic, and diastolic parameters computed, as described earlier [20]. Renal function, proteinuria, retinopathy, and neuropathy were evaluated, as detailed previously [14, 15, 20-22].
Statistical analysis
Data are presented as mean ± standard deviation (SD). Post-transplantation survival data were calculated by Kaplan-Meier analysis. Comparisons of data were performed using Student's t-test for paired data, or chi-square test.
Results
Patient and pancreas survival
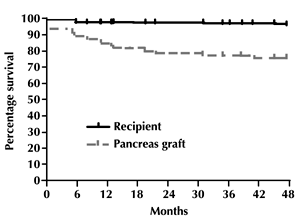
As shown in Figure 1, Kaplan-Meier survival analysis showed that patient and pancreas (full insulin-independence) survivals were respectively 98.4% and 85.5% at 1 yr, 98.4% and 79.7% at 2 yr, 98.4% and 78.3% at 3 yr, and 98.4% and 76.7% at 4 yr. One patient died 5 months after transplant due to disseminated CMV disease. No patient developed cardiovascular events, or morbidities, other than those reported in this and the following paragraphs. The actual 4-year rates for patient and pancreas survival (24 patients with this follow-up) were 98.2% and 77.1%, respectively. Among patients with a functioning pancreas graft at 1 yr, 88.7% were still insulin-independent at the 4-year follow-up control.
 |
 |
Figure 1. Kaplan-Meier curves showing patient and graft survival up to 4 years after PTA. |
|
Overall, 10 recipients developed vascular thrombosis, with occlusions occurring in 3 of them. No vein thrombosis extended beyond the site of anastomosis between donor and recipient vessels. Thus, no patient needed caval, or portal, thrombectomy.
Repeat surgery was necessary in 13 patients (18.2%). In detail, relaparotomy was required because of bleeding (6 recipients, 8.4%), occlusive vascular thrombosis (3 recipients, 4.2%), hyperacute rejection (3 recipients, 4.2%), and duodenal graft anastomotic leak (1 recipient, 1.4%). Clinically relevant infections (requiring assistance from our center) developed in 11 patients (15.5%), 5 were bacterial and 6 CMV infections (including the fatal case mentioned above).
Three patients experienced hyperacute rejection. Diagnosis was confirmed after allograft pancreatectomy through histological findings and a sudden rise in donor-specific antibodies. Fifteen acute rejection episodes were recorded in 14 patients:
- 2 recipients experienced acute rejection in the first month post-transplantation,
- 1 recipient after 72 days,
- 5 recipients between 3 and 6 months post-PTA,
- 2 recipients between 6 months and 1 year, and
- 4 recipients later on.
In these patients, rejection was suspected because of a more than two-fold elevation in pancreatic enzymes in the absence of other possible explanations. Rejection was biopsy-proven in all patients, graded according to Drachenberg et al. [23], and successfully treated (excluding the 3 hyperacute episodes) with a 10 day course of monoclonal antibody therapy [24]. During the follow-up, 12 recipients were eventually diagnosed with chronic allograft rejection. Seven of them were previously diagnosed with one (n = 6), or two (n = 1), episodes of acute rejection. All patients presented deteriorating metabolic control, but usually with some degree of residual function (C-peptide range, 0.8 to 2.3 ng/ml). Diagnosis was based on pancreas biopsy and/or evidence of persisting donor-specific antibodies.
Effects on glycemic control and kidney function
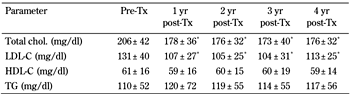
Figure 2 shows fasting plasma glucose, HbA1c, and fasting C-peptide concentrations before transplantation and at 1, 2, 3, and 4 years post-transplantation in patients with functioning grafts. Normalization of glucose values without exogenous insulin administration was rapidly achieved, and solidly maintained throughout the study period in all successful cases. Total and LDL-cholesterol levels decreased significantly after transplantation without change in HDL-cholesterol and triglyceride levels (Table 1). These results were achieved without major changes in the use of statins (18.6% before transplantation vs. 26% at 4 years). Prior and 4-year systolic and diastolic blood pressure values (mmHg) were 129 ± 10 and 115 ± 6 (p < 0.01), and 82 ± 8 and 73 ± 7 (p < 0.01) respectively. Any antihypertensive treatment (ACE inhibitors in 92% of cases) before transplantation (42%) was maintained until the end of the 4-year follow-up (50%).
 |
 |
Figure 2. Glycemic indices (fasting plasma glucose, A, and HbA1c, B) and fasting C-peptide levels (C) before and after pancreas transplantation. Tx: transplantation. |
|
Table
1.
Lipid parameters before and after PTA |
|
 |
 |
Legend:
Data are mean ± SD. Student's t-test was used for statistical calculations. C: cholesterol. HDL: high-density lipoprotein. LDL: low-density lipoprotein. PTA: pancreas transplantation alone. TG: triglyceride. * p < 0.05. |
|
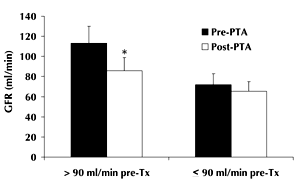
Overall, proteinuria decreased from 1.36 ± 2.72 g/day (pre-transplant) to 0.29 ± 0.51 g/day (last control post-transplant; p < 0.01). Serum creatinine concentrations increased from 0.95 ± 0.28 to 1.17 ± 0.26 mg/dl (p < 0.01). GFR, calculated according to the Cockroft-Gault formula, decreased by approximately 20% from 94 ± 39 to 75 ± 22 ml/min (p < 0.01). Further analyses were performed in patients who reached the 4-year follow-up examination, based on the pre-transplant GFR, > or ≤ 90 ml/min (Figure 3). Renal function decreased significantly in patients with a GFR above 90 ml/min, whereas GFR remained substantially unchanged in patients with GFR below 90 ml/min.
 |
 |
Figure 3. Estimated glomerular filtration rate (GFR) before and after pancreas transplantation alone (PTA) according to pre-transplantation values. * p < 0.01. |
|
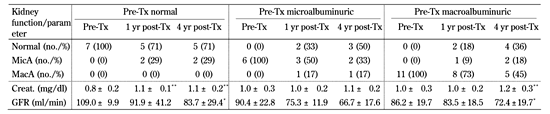
A similar analysis was performed depending on the presence or absence of proteinuria (Table 2). In non-albuminuric patients, 2 developed microalbuminuria, and kidney function declined significantly over time, with an overall average GFR decrease of 17 ml/min in the first year, and 2.7 ml/min/year in the follow-up. Of the 6 patients with microalbuminuria prior to transplantation (daily urinary protein excretion 0.23 ± 0.05 g), 3 patients became normoalbuminuric, 2 patients remained microalbuminuric, and 1 subject developed macroproteinuria. Their creatinine levels and GFR showed evidence of deterioration, but without reaching statistical significance (Table 2). In this group, GFR decreased by 15.1 ml/min during the first year, and 2.87 ml/min per year thereafter. Eleven patients were macroproteinuric (2.97 ± 3.55 g/day) before transplantation. At the end of the 4-yr follow-up period, more than half of them showed reduction or even disappearance of proteinuria (Table 2). GFR decrease in these patients was slow (3.4 ml/min per year), although significant at 4 years post-transplantation.
Table
2.
Kidney function follow-up according to pre-transplant proteinuria status |
|
 |
 |
Legend:
Data are mean ± SD. Student's t-test was used for statistical calculations. Creat.: creatinine. GFR: glomerular filtration rate. MicA: microalbuminuric. MacA: macroalbuminuric. No.: number. Tx: transplantation. * p < 0.05 vs. pre-Tx. ** p < 0.01 vs. pre-Tx. |
|
Effects on cardiac function, retinopathy, and neuropathy
Cardiac parameters, assessed by doppler echocardiographic examinations, were within the normal range, and similar to those reported for a local control population at the pre-transplant evaluation (data not shown). At the end of the post-transplantation follow-up period, left ventricular ejection fraction increased slightly, but significantly, from 54.4 ± 4.3% to 57.4 ± 3.2% (p < 0.01), and the E/A velocity ratio (a diastolic parameter obtained by doppler mitral flow) also improved (from 1.18 ± 0.33 to 1.38 ± 0.50 cm/sec), although not significantly. The other indices remained stable (data not shown).
According to the grading methods, and the results previously reported at an earlier follow-up control [14], PTA had beneficial effects on diabetic retinopathy. Before transplantation, 7.5% of patients had no retinopathy, and all of them remained lesion-free at 4 years. Of the 29.5% patients with non-proliferative retinopathy, 75% improved and 25% remained unchanged. Finally, in the group of patients with proliferative and/or laser-treated retinopathy, lesions remained stable in 82% of patients and progressed to a more serious grade in the remaining 18%.
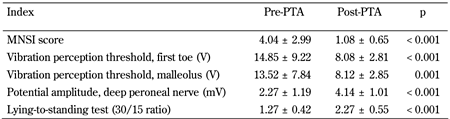
As shown in Table 3, neuropathy assessment showed a significant improvement in several indices of peripheral and autonomic responses after PTA.
Table
3.
Neuropathy assessment before and after PTA |
|
 |
 |
Legend:
Data are mean ± SD. Student's t-test was used for statistical calculations. |
|
Discussion
We have reported on a single center experience with PTA focusing on the effects of PTA up to 4 years after transplantation. We assessed metabolic parameters, cardiovascular risk factors, renal function and proteinuria (i.e. diabetic nephropathy), and chronic diabetic complications. Patient survival and insulin independence rates are similar to, or even slightly better than, those reported in other contemporary series [1-3]. Our improved outcomes may in part be explained by a close follow-up, mostly carried out at our institution. It confirms that current PTA regimens are largely safe (in terms of patient survival) and effective (in terms of graft function).
Normalization of plasma glucose concentrations was solid and sustained, as documented by HbA1c level stability within normal ranges. Moreover, improvement of several cardiovascular risk factors, previously reported after a shorter follow-up period [20], was maintained after 4 years post-PTA. Non-significant 14% and 8% declines in LDL-cholesterol, 1 and 2 years after PTA, have been previously reported in a small group of patients (n = 11) with systemic venous drainage of endocrine secretion of the graft [25]. It remains unclear whether the portal or the systemic drainage of insulin may further improve lipid levels [26, 27], but it is a fascinating hypothesis based on solid theoretical grounds.
We found a significant decrease in proteinuria levels after PTA and a 20% cumulative decrease of GFR over the 4 years of follow-up. Similar rates of GFR loss after 5 years post-PTA have been recently reported, with no major difference, irrespective of which calcineurin inhibitor was used [17]. Interestingly, in the present study, the loss of renal function after PTA was less significant in patients with lower baseline GFR (< 90 ml/min) than in patients with higher pristine renal function (GFR > 90 ml/min). Possibly, patients from the latter group were already in the stage of hyperfiltration at the time of PTA, and this seemed to result in higher renal function. Should this interpretation be correct, a moderate GFR decline after PTA could actually mean improvement of renal function as a result of correction of hyperfiltration. Renal histology could not be performed routinely before or after PTA as our institutional policy does not favor protocol biopsies. Nevertheless, it is known that long-term normoglicemia (> 10 years) improves renal histology after PTA, despite GFR reduction from a mean of 108 ± 20 ml/min/1.73 m2 before PTA to 74 ± 14 ml/min/1.73 m2 10 years after PTA [28, 29]. Therefore, our observation could either mirror an early stage of this process that is possibly not yet evident in histology, or it could identify a subgroup of patients in whom normalization of metabolic control results in earlier clinical benefit.
Regarding the question why proteinuria declined after PTA, it is possible that normalization of blood glucose and restored C-peptide secretion may play a role, as previously discussed [15]. Reduction in glucose levels is associated with decreased hyperfiltration and diminished albuminuria [30, 31]. C-peptide exerts beneficial actions on the endothelium in the diabetic kidney [32]. The effects of PTA on the reduction of proteinuria may be of particular relevance. Notably, very recent data show that the risk of end-stage renal disease remains high and unchanged despite reno-protective treatments [33]. This finding may suggest the need for new therapies in diabetic patients with overt diabetic nephropathy.
It is conceivable that the normoglycemic condition and the improvements in other parameters (lipids and blood pressure) have contributed to the mitigation of chronic diabetic complications. Evidence for improved ventricular ejection fraction, as assessed by echocardiography, confirmed our initial report in fewer patients with shorter follow-up [20]. It should however be noted that echocardiography parameters were mostly normal before PTA. This is possibly due to stringent candidate selection and an earlier indication to PTA, as compared to SPK. Consequently, no conclusion can be drawn on the question whether improvements in the already satisfactory cardiac function in PTA recipients translates into the same survival benefits seen in SPK recipients [1-3].
In a report dealing with a follow-up of approximately 2 years, it was observed that diabetic retinopathy improved, or stabilized, in the majority of T1D patients after PTA, with a substantial benefit against non-transplanted subjects [14]. Our study confirms these results and shows that improvement in retinopathy is maintained at the longer follow-up period of 4 years, as already observed after SPK [34, 35]. Also, our series of PTA confirms the positive impact on diabetic neuropathy, as previously reported after SPK, PAK, and PTA [36]. This was the case for both peripheral and autonomic lesions. These observations have not been made with current intensive insulin treatment [37].
Overall, the improvements seen in the clinical course of diabetic complications indicate that normalization of metabolic control improves microvascular complications in PTA recipients. Basically, this should mean that PTA recipients have a longer life expectancy than non-transplanted patients continuing on exogenous insulin supply, despite similar baseline medical conditions. Demonstration of this hypothesis would require a well-designed prospective randomized comparison. The most reliable information that is currently available comes from two independent retrospective analyses of the UNOS data base. Interestingly, small differences in methodology lead to opposite conclusions. In the first of these surveys, Venstrom et al. reported an increased risk of mortality for PTA recipients (RR 1.57 at 4 years), as compared to patients on the wait list [5]. In the second survey, Gruessner et al. [6-8] extended the follow-up period, excluded patients listed at multiple centers, and included deaths occurred shortly after withdrawal from the wait list. In this analysis, the authors could not confirm the increased risk of mortality for PTA recipients.
Hopefully, answers to the many pending questions surrounding PTA may eventually be provided by the recently implemented PANCREAS study, a prospective multi-institution study randomizing selected T1D patients to PTA, or intensive insulin. treatment [38]. Until new data are available, current information suggest that PTA may be a beneficial option in selected T1D patients. This seems to be particularly true when considering T1D patients with established markers of decreased survival such as neuropathy, severe retinopathy, or proteinuria. Since renal function is the key in all diabetics, PTA candidates should have an acceptable baseline renal reserve (GFR > 60/70 ml/min) [1-3].
Disclosures: The authors report no conflict of interests.
References
- Larsen JL. Pancreas transplantation: indications and consequences. Endocr Rev 2004. 25:919-946. [DOD] [CrossRef]
- Gruessner AC, Sutherland DE, Gruessner RW. Pancreas transplantation in the United States: a review. Curr Opin Organ Transplant 2010. 15:93-101. [DOD] [CrossRef]
- White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet 2009. 373:1808-1817. [DOD] [CrossRef]
- Schenker P, Vonend O, Krüger B, Klein T, Michalski S, Wunsch A, Krämer BK, Viebahn R. Long-term results of pancreas transplantation in patients older than 50 years. Transpl Int 2011. 24:136-142. [DOD] [CrossRef]
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003. 290(21):2817-2823. [DOD] [CrossRef]
- Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud 2011. 8(1):6-16. [DOD] [CrossRef]
- Gruessner RW, Sutherland DE, Gruessner AC. Survival after pancreas transplantation. JAMA 2005. 293:675-676. [DOD] [CrossRef]
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004. 4:2018-2026. [DOD] [CrossRef]
- Dean PG, Kudva YC, Stegall MD. Long-term benefits of pancreas transplantation. Curr Opin Organ Transplant 2008. 13:85-90. [DOD] [CrossRef]
- Gremizzi C, Vergani A, Paloschi V, Secchi A. Impact of pancreas transplantation on type 1 diabetes-related complications. Curr Opin Organ Transplant 2010. 15:119-123. [DOD] [CrossRef]
- American Diabetes Association. Pancreas and islet transplantation. Diabetes Care 2006. 29:935. [DOD] [CrossRef]
- Societa Italiana di Diabetologia. Il trapianto di pancreas e di isole pancreatiche. Il Diabete 2002. 14:113-116. [DOD]
- Gruessner RW, Sutherland DE, Kandaswamy R, Gruessner AC. Over 500 solitary pancreas transplants in non-uremic patients with brittle diabetes mellitus. Transplantation 2008. 85:42-47. [DOD] [CrossRef]
- Giannarelli R, Coppelli A, Sartini MS, Del Chiaro M, Vistoli F, Rizzo G, Barsotti M, Del Prato S, Mosca F, Boggi U, Marchetti P. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia 2006. 49(12):2977-2982. [DOD] [CrossRef]
- Coppelli A, Giannarelli R, Vistoli F, Del Prato S, Rizzo G, Mosca F, Boggi U, Marchetti P. The beneficial effects of pancreas transplant alone on diabetic nephropathy. Diabetes Care 2005. 28(6):1366-1370. [DOD] [CrossRef]
- Scalea JR, Butler CC, Munivenkatappa RB, Nogueira JM, Campos L, Haririan A, Barth RN, Philosophe B, Bartlett ST, Cooper M. Pancreas transplant alone as an independent risk factor for the development of renal failure: a retrospective study. Transplantation 2008. 86(12):1789-1794. [DOD] [CrossRef]
- Fioretto P, Najaran B, Sutherland DE, Mauer M. Tacrolimus and cyclosporine nephrotoxicity in native kidneys of pancreas transplant recipients. Clin J Am Soc Nephrol 2011. 6(1):101-106. [DOD] [CrossRef]
- Boggi U, Amorese G, Marchetti P. Surgical techniques for pancreas transplantation. Curr Opin Organ Transplant 2010. 15:102-111. [DOD] [CrossRef]
- Boggi U, Vistoli F, Del Chiaro M, Signori S, Pietrabissa A, Costa A, Bartolo TV, Catalano G, Marchetti P, Del Prato S, et al. A simplified technique for the en bloc procurement of abdominal organs that is suitable for pancreas and small-bowel transplantation. Surgery 2004. 135(6):629-641. [DOD] [CrossRef]
- Coppelli A, Giannarelli R, Mariotti R, Rondinini L, Fossati N, Vistoli F, Aragona M, Rizzo G, Boggi U, Mosca F, Del Prato S, Marchetti P. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation 2003. 76(6):974-976. [DOD] [CrossRef]
- Giannarelli R, Coppelli A, Sartini M, Aragona M, Boggi U, Vistoli F, Rizzo G, Del Prato S, Mosca F, Marchetti P. Effects of pancreas-kidney transplantation on diabetic retinopaty. Transpl Int 2005. 18(5):619-622. [DOD] [CrossRef]
- Piaggesi A, Castro Lopez E, Bini L, Benzi L, Giampietro O, Schipani E, Navalesi R. Measurable deficit of autonomic and sensory nerve function in asymptomatic diabetic patients. J Diabetes Complications 1992. 6(3):157-162. [DOD] [CrossRef]
- Drachenberg CB, Odorico J, Demetris AJ, Arend L, Bajema IM, Bruijn JA, Cantarovich D, Cathro HP, Chapman J, Dimosthenous K, Fyfe-Kirschner B, et al. Banff schema for grading pancreas allograft rejection: working proposal by a multi-disciplinary international consensus panel. Am J Transplant 2008. 8(6):1237-1249. [DOD] [CrossRef]
- Gruessner RW. Immunobiology, diagnosis, and treatment of pancreas graft rejection. In: Gruessner RW, Sutherland DE (eds.). Transplantation of the pancreas. Springer-Verlag, New York, 2004, pp. 349-380. [DOD]
- Lauria MW, Figueiro JM, Machado LJ, Sanches MD, Lana AM, Ribeiro-Oliveira A Jr. The impact of functioning pancreas-kidney transplantation and pancreas alone transplantation on the lipid metabolism of statin-naive diabetic patients. Clin Transplant 2009. 23(2):199-205. [DOD] [CrossRef]
- Martin X, Petruzzo P, Dawahra M, Feitosa Tajra LC, Da Silva M, Pibiri L, Chapuis F, Dubernard JM, Lefrancois N. Effects of portal versus systemic venous drainage in kidney-pancreas recipients. Transpl Int 2000. 13(1):64-68. [DOD] [CrossRef]
- Petruzzo P, Laville M, Badet L, Lefrancois N, Bin-Dorel S, Chapuis F, Andreelli F, Martin X. Effects of venous drainage on insulin action after simultaneous pancreas-kidney transplantation. Transplantation 2004. 77(12):1875-1879. [DOD] [CrossRef]
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998. 339(2):69-75. [DOD] [CrossRef]
- Fioretto P, Sutherland DE, Najafian B, Mauer M. Remodelling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int 2006. 69(5):907-912. [DOD] [CrossRef]
- Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes 2001. 109(Suppl 2):424-437. [DOD] [CrossRef]
- Remuzzi A, Viberti G, Ruggenenti P, Battaglia C, Pagni R, Remuzzi G. Glomerular response to hyperglycemia in human diabetic nephropathy. Am J Physiol 1990. 259(4 Pt 2):F545-F552. [DOD]
- Nordquist L, Wahren J. C-Peptide: the missing link in diabetic nephropathy? Rev Diabet Stud 2009. 6(3):203-210. [DOD] [CrossRef]
- Rosolowsky ET, Smiles AM, Skupien J, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS. The risk of ESRD in patients with Type 1 diabetes and macroalbuminuria remains high despite reno-protective treatment. J Am Soc Nephrol 2011. 22(3):545-553. [DOD] [CrossRef]
- Shipman KE, Patel CK. The effect of combinated renal and pancreatic transplantation on diabetic retinopathy. Clin Ophthalmol 2009. 3:531-535. [DOD] [CrossRef]
- Koznarova R, Saudek F, Sosna T, Adamec M, Jedinakova T, Boucek P, Bartos V, Lanska V. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant 2000. 9:903-908. [DOD]
- Navarro X, Sutherland DE, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol 1997. 42:727-736. [DOD] [CrossRef]
- Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Circulation 2009. 119(22):2886-2893. [DOD] [CrossRef]
- Pancreas Allotransplantation for Diabetic Nephropathy and Mild Chronic REnal fAilure Stage Study (PANCREAS). http://clinicaltrials.gov/ct2/show/NCT01067950. [DOD]
This article has been cited by other articles:

|
Pancreas transplantation: a treatment option for people with diabetes
Mittal S, Gough SC
Diabet Med 2014. 31(5):512-521
|
|

|
Microangiopathy is common in submucosal vessels of the colon in patients with diabetes mellitus
Sasor A, Ohlsson B
Rev Diabet Stud 2014. 11(2):175-180
|
|
|
The therapeutic potential of C-peptide in kidney disease: a protocol for a systematic review and meta-analysis
Shaw J, Shetty P, Burns K, Knoll G
Syst Rev 2014. 3:43
|
|
|
Simultaneous kidney and pancreas transplantation in patients with type 1 diabetes mellitus at Clinica Fundacion Valle del Lily, Cali, Colombia
Serrano OJ, Villegas JI, Echeverri GJ, Posada JG, Mesa L, Schweineberg J, Duran C, Caicedo LA
Rev Colomb Cir 2014. 29:32-41
|
|

|
Pancreas transplant alone: a procedure coming of age
Gruessner RW, Gruessner AC
Diabetes Care 2013. 36(8):2440-2447
|
|

|
Follow-up of secondary diabetic complications after pancreas transplantation
Boggi U, Rosati CM, Marchetti P
Curr Opin Organ Transplant 2013. 18(1):102-110
|
|

|
The metabolic and toxicological considerations for immunosuppressive drugs used during pancreas transplantation
Rangel EB
Expert Opin Drug Metab Toxicol 2012. 8(12):1531-1548
|
|

|
Evolution of pancreas transplantation: long-term results and perspectives from a high-volume center
Ollinger R, Margreiter C, Bösmüller C, Weissenbacher A, Frank F, Schneeberger S, Mark W, Margreiter R, Pratschke J
Ann Surg 2012. 256(5):780-786
|
|

|
Transplantation of the pancreas
Boggi U, Vistoli F, Egidi FM, Marchetti P, De Lio N, Perrone V, Caniglia F, Signori S, Barsotti M, Bernini M, Occhipinti M, Focosi D, Amorese G
Curr Diab Rep 2012. 12(5):568-579
|
|
|
In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation
Tang DQ, Wang Q, Burkhardt BR, Litherland SA, Atkinson MA, Yang LJ
Am J Stem Cells 2012. 1(2):114-127
|
|
|