Diabetic Perspectives
| Rev Diabet Stud,
2007,
4(1):56-61 |
DOI 10.1900/RDS.2007.4.56 |
The Development and Application of HLA Tetramers in the Detection, Characterization and Therapy of Type 1 Diabetes Mellitus
Hsin-Wei Chen, Shih-Jen Liu, Pele Chong, Charles Sia
Vaccine Center, National Health Research Institutes, 35 Keyan Road, Zhunan Township, Miaoli County, Taiwan.
Address correspondence to: Charles Sia, e-mail: siady@nhri.org.tw
Keywords: type 1 diabetes, HLA tetramers, T cell, islet antigen
Abstract
Islet antigens are presented by human leukocyte antigen (HLA) class I and II molecules and are recognized by CD8+ and CD4+ autoreactive T cells in type 1 diabetic individuals. Early identification of individuals at risk for the disease by detection of these antigens and the autoreactive cells themselves is essential for understanding pathogenesis and for intervention at an early stage to prevent ongoing beta-cell destruction. However, the methods of identifying autoimmune development at an early stage have appeared to be limited because of the heterogeneity of the disease. The appearance of autoantibodies in preclinical type 1 diabetes mellitus (T1DM) does not follow specific patterns and depends on patient characteristics such as age. Also, results obtained with cytokine assays revealed that the number of islet antigen-responsive T cells present in the pool of peripheral blood mononuclear cells (PBMC) of non-diabetic individuals is highly variable and can be similar to that assayed in diabetics. Therefore, new identification and detection methods are needed. In this context, the use of HLA epitopes to generate stable HLA epitope tetramers has recently proved to be a promising approach to the detection of autoreactive T cells in antigen-stimulated PBMC cultures from diabetic and pre-diabetic subjects. HLA class II tetramers have been found to be capable not only of detecting TCRαβ of different avidities for a common ligand, e.g. GAD65555-567(mimitope), but also of inducing apoptosis in lymphocytes with high TCRαβ avidity for this ligand. This observation even opens up a potential application of HLA class II tetramers as therapeutic agents for immune intervention in T1DM.
Introduction
Type 1 diabetes mellitus (T1DM) is an organ-specific autoimmune disease characterized by the destruction of pancreatic β-cells by an inflammatory process involving diabetogenic T lymphocytes, autoantibodies and innate immune mechanisms. The T cell-mediated attack results in the development of overt T1DM causing continuous dependence on insulin therapy. Diabetes onset is preceded by a preclinical stage which can be highly variable as regards the types of autoantibodies generated against islet antigens and their appearance and duration in the serum during prediabetes [1]. Another characteristic of autoantibodies in preclinical T1DM is that their appearance and duration do not follow specific patterns. Assay improvements made over the years have led to greater specificity and sensitivity in the detection of antibodies against glutamic decarboxylase (GAD), insulin-associated protein IA-2, a tyrosine phosphatase-like protein and insulin. The use of these parameters has enabled clinicians to identify individuals at high risk of the disease [2].
Despite this development, several criteria have imposed limitations on the application of islet antigen-specific antibody measurements as a universal prediction tool for T1DM onset. No direct and clear-cut correlation has been observed between autoantibody appearance and T1DM initiation and development. Autoantibodies are detected in the serum of infants of antibody-positive mothers in the absence of diabetes in the child [3]. On the other hand, high antibody levels against GAD are detected in some individuals whose pancreatic islets are found to have been almost completely destroyed [4]. Therefore, another technique is required in order to detect autoimmune initiation at an earlier stage of disease development. Such an option would enable earlier immune-interventions to halt the immune process before it leads to overt clinical disease. Early intervention opens up a window for antigen-specific therapy, which is able to induce long-term tolerance through the activation of regulatory T cells. Consequently, earlier intervention provides a better chance of restoring islet function.
Another critical finding, which complicates the identification of individuals at risk of developing the disease, is that the appearance of autoantibodies to islet antigens, such as GAD and insulin, has been considered to be significantly influenced by the individual’s age. In a Japanese population, antibodies to these antigens have been found to be undetectable in a significant number of patients with T1DM. The antibodies to GAD and other antigens showed an age-at-onset dependent segregation in this study. The GAD autoantibody did not complement other antibodies acting as markers of T1DM in the group made up of very young children (0-5), but was a more indicative marker in the >13 years group [5]. The current method of identifying individuals at risk by the use of antibody titers alone is therefore insufficient. Other methods are required to improve monitoring of the prediabetic stage
A different approach is the detection of circulatory islet antigen-specific T cells which is based on the observation that the pathogenesis of T1DM is accompanied by a direct infiltration of autoreactive T cells into the pancreatic islets [6, 7]. Many attempts have been made to develop a more representative index of β-cell destruction based on this approach. Initial detection methods have involved in vitro antigen-stimulation of islet antigen-specific precursor T cells isolated from peripheral blood mononuclear cells (PBMC) of diabetic subjects followed by measurement of the proliferation and/or cytokine release of expanded autoreactive T cell populations. However, further examination showed that results from these studies are inconsistent. This is primarily due to the stimulatory property of endotoxins present in the recombinant islet antigen preparations that have been added to the cultures [8].
Another version of the cytokine assays is the enzyme-linked immunosorbent spot (ELISPOT) that has been employed to detect the frequencies of islet antigen-specific T cells in the peripheral blood of diabetic subjects. Results obtained with this assay revealed that the number of islet antigen-responsive T cells present in the pool of PBMCs of non-diabetic individuals is highly variable and can be similar to that assayed in diabetics [9, 10]. This can again be attributed to endotoxin-contaminated islet antigen preparations which are used in the in vitro cultures. Recently, a more sensitive ELISPOT assay used with immunoglobulin-free culture medium has been described, which showed a more reliable detection of GAD65-specific autoreactive T cells in diabetic patients [11]. Given that T lymphocytes activated via engagement of their αβ T cell receptor (TCRαβ) together with their antigenic ligand in association with the respective major histocompatibility complex (MHC) class I and II molecules could either undergo proliferation or antigen-induced cell death (ACID) [12], it is reasonable to suppose that these events can bias the detection of islet antigen-specific T cells. Furthermore, the presence of natural T regulatory (Treg) cells that suppress T lymphocytes responding to islet antigens can interfere with the detection of autoreactive T cells in in vitro PBMC cultures [13]. Despite these complications, the use of in vitro PBMC culture protocols has allowed the identification of some epitopes of several islet antigens, such as GAD, proinsulin/insulin and human islet amyloid polypeptide (preprolAPP), presented in the context of T1DM-susceptible human leukocyte antigen (HLA) alleles. These findings are discussed in more detail in subsequent sections.
A clear understanding of the interaction of the TCR with its cognate MHC/peptide ligand, followed by successes in preparing soluble MHC proteins that physically associate with the respective epitopes, has led to the development of tetrameric MHC/peptide complexes capable of binding to antigen-specific T cells via their TCRαβ. Figure 1 shows in general terms how these tetramers are made and work. The first tetrameric MHC/peptide complexes with sufficient avidity to facilitate the ex vivo detection of antigen-specific T cells by flow cytometry were reactive with T cells specific for foreign antigens [14]. In T1DM, it has been suggested that the priming process, whereby autoreactive T cells infiltrate pancreatic islets, involves activation of CD4+ lymphocytes through recognition of islet antigen peptides, which have been presented by local antigen-presenting cells. The immunological help provided by the autoreactive CD4+ T cells then initiates the activation of islet antigen-specific CD8+ T cells [15]. On this basis, the detection of peripheral CD4+ T cells reactive to islet antigens has been explored in order to develop an early index that reflects the beginning of β-cell destruction.
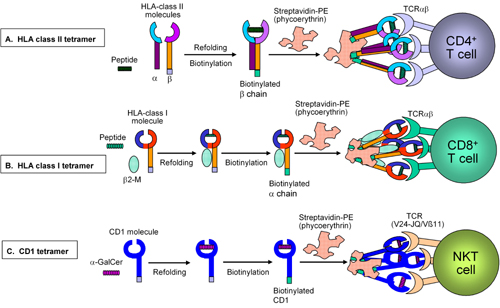 |
 |
Figure 1. Preparation of HLA class I, II and CD1 tetramers. Soluble recombinant HLA class I and II or CD1 molecules are refolded to incorporate the respective antigen ligands: usually > 10 amino acid long peptide for HLA class II (e.g. from GAD65) (A); 9-10 amino acid long peptide for HLA class I (e.g. from insulin β chain or GAD65) (B) and α-GalCer (artificial ligand) for CD1 (C). The respective tetramers are then generated via association of the biotinylated HLA class I/II/CD1 molecules with one molecule of fluorochrome-conjugated streptavidine. Subsequently, specific T cells bind to the respective HLA/peptide or CD1/ligand complex via simultaneous engagement of three TCRs each to establish stable and specific binding to the T cell. |
|
Tetramer detection of HLA class II-restricted autoreactive T cells in diabetic subjects and its implications
Tetramers constructed with high risk HLA class II molecules incorporating the respective epitopes of GAD65 and proinsulin have been developed to stain autoreactive CD4+ T cells in diabetic subjects (Figure 1A). Following in vitro antigen stimulation of unfractionated PBMCs, which enriches the respective population of autoreactive T cells, a population of lymphocytes bearing the CD4+CD25+ phenotype is found to be present in 40-60% of HLA-DR*04+ diabetic individuals and at risk subjects. The specificity of these CD4+CD25+ Treg cells has been demonstrated by their binding to a HLA-DR*0401/*0404-GAD65555-567(mimitope) tetramer that contains an altered epitope (mimitope) involving an F to I substitution required for its stable association with the HLA class II molecule [16, 17]. Interestingly, similar antigen enrichment of PBMC cultures from non-diabetic HLA-DR*04+ subjects depleted of CD4+CD25+ Treg cells also results in the generation of an increased number of HLA-DR*0401/*0404-GAD65555-567(mimitope)-binding lymphocytes [18]. This implies that autoreactive T cells can also be present in non-diabetic individuals who, nevertheless, remain healthy because they have a sufficient number of functional and suitable Treg cells in their blood, which suppress autoimmune reactions. Another tetramer, HLA-DR*0401-GAD65274-286, detects GAD65274-286-specific T cells in these groups of HLA-DR*04+ diabetic subjects as well. In individuals who carry the disease-associated HLA-DR*0301 allele, a HLA-DR*0301-proinsulin(B24-36) tetramer detects the presence of proinsulin(B24-36)-reactive T cells in their PBMCs [17].
Apart from the detection of autoreactive T cells, results obtained from tetramer staining studies have allowed several inferences to be drawn with respect to the association of these lymphocytes with T1DM. First, it enables the frequencies of different islet antigen-specific T cell populations to be estimated. This also requires the use of a CSFE-based proliferation assay, which makes identification of cell divisions in response to antigen stimulation possible. This method is based on detecting and counting the number of cell divisions that are directly correlated with the serial dilution of the dye incorporated in the cells at culture initiation [19]. With this assay, it is possible to determine if autoreactive T cells are present in the circulation by antigen-stimulation of PBMC following CFSE-labeling. However, the presence of GAD65555-567-reactive cells is estimated to be no more than 1 in 30,000 in circulation at any one time [17], which makes it difficult to detect antigen-specific T cells directly ex vivo. Furthermore, autoreactive T cells stained with a given tetramer, for example HLA-DR*0401/*0404-GAD65555-567(mimitope), are comprised of lymphocytes expressing TCRαβ with different avidity against the multimeric HLA class II/epitope complex. High avidity HLA-DR*0401/*0404-GAD65555-567(mimitope)-binding cells respond to mimitope stimulation and undergo greater proliferation and IFN-γ production as compared to the low avidity tetramer-reactive cells [20]. Apparently, antigen-stimulation of high avidity cells would eventually drive them to undergo AICD, while low avidity lymphocytes are resistant to progress along this pathway upon subjection to the same stimulatory condition. Collectively, results obtained from these studies reveal that HLA class II tetramers warrant testing out as potential tolerogens for milieux containing high avidity TCRαβ-bearing islet antigen-specific CD4+ cells. This would leave the development of a separate strategy for low avidity autoreactive T cells, which is needed to halt ongoing T cell-associated autoimmunity.
Tetramer detection of HLA class I-restricted autoreactive T cells in diabetic subjects and its implications
In contrast to the use of HLA-class II tetramers, which makes it possible to estimate the frequencies of islet antigen-specific lymphocytes as well as their isolation for further characterization, the application of HLA class I tetramers in the analysis of CD8+ autoreactive T cells in diabetic subjects is limited. A recent study describes the combined use of a HLA class I-binding motif prediction algorithm with the analysis of peptides derived from proteasome digest of insulin β chain in identifying a putative HLA-A0201-restricted epitope (insB10-18), which encompasses amino acid residues 10-18 of the insulin β chain. (insB10-18) binds empty HLA-A0201 molecules expressed on TAP-defective T2 cells and stabilizes them. A tetramer designated HLA-A2insB10-18, containing the peptide (insB10-18) associated with the HLA-A0201 molecule, stains CD8+ T lymphocytes generated from in vitro PBMCs of non-diabetic individuals that have been subjected to repeated stimulation with the insB10-18 peptide (Figure 1B). HLA-A2insB10-18-binding cells have been shown to have a cytolytic phenotype as judged by their production of IFN-γ and granzyme B following stimulation with insB10-18, As is the case with the presence of GAD65555-567-reactive lymphocytes in non-diabetic individuals, these findings imply that CD8+ autoreactive T cells, such as those directed against insB10-18, also exist in healthy individuals but at a very low frequency [21]. For this reason and perhaps also because of the kinetics of insB10-18-reactive cells appearing in the circulation, HLA-A2insB10-18 has failed to detect autoreactive T cells specific to this epitope in diabetic subjects. On the other hand, HLA-A2insB10-18-binding lymphocytes are found in the PBMC pool of HLA-A0201-positive patients with ongoing autoimmunity that is associated with rejecting HLA-A0201-matched islet transplants.
Reports on the broader characterization of autoreactive T cells detected by HLA class I tetramers are currently lacking, but studies using in vitro antigen-stimulation protocols have progressed to identify lymphocytes in the PBMC pools of diabetic subjects that respond to epitopes in different islet cell antigens. These antigens include GAD65 [22] and the leader sequence of preproIAPP presented in the context of HLA-0210 molecules [23]. In the case of preproinsulin and proinsulin they contain several epitopes that stimulate CD8+ T cells in diabetic subjects who carry HLA-class I alleles other than HLA-A0201 [24, 25]. On the basis of these findings can be expected that more HLA class I tetramers could detect islet antigen-specific CD8+ T cells in individuals with T1DM. The recent observation that blocking of the CD8 co-receptor is required to enhance HLA-class I tetramer binding to T cells warrants the incorporation of this approach in the tetramer staining protocol for the detection of autoreactive CD8+ T cells in diabetics [26].
CD1d tetramers in the detection of natural killer T (NKT) cells in diabetic subjects
Several studies have investigated the role of NKT cells that express an invariant TCR, Vα24-Jα18/Vβ11, in the development of T1DM. Studies conducted with diabetic subjects from different geographical regions showed that lymphocytes in the PBMC pools of these individuals stained by monoclonal antibodies against Vα24 and Vβ11 can either be reduced or increased [27, 28]. Functional studies have suggested that the impairment of IL-4 secretion occurs in Vα24+Vβ11+ TCR-bearing cells in T1DM patients [29]. Another study made use of a CD1d tetramer, which contains its artificial ligand, αGalCer, to detect Vα24-Jα18/Vβ11+ cells (Figure 1C). It turned out that the appearance of these cells is comparable among patients, individuals at risk and healthy subjects [30]. While there is, as yet, no consensus about the role of NKT cells in relation to T1DM, the application of tetramers in the detection of NKT cells has contributed to knowledge of their frequencies and function in T1DM.
Conclusion
In summary, HLA class II tetramers are able to detect autoreactive CD4+ T cells in islet antigen-stimulated PBMC cultures. The application of these tetramers could therefore contribute to frequency estimations of autoreactive lymphocytes in diabetic subjects. More data collected from studies with new HLA tetramers may help to evaluate the number of autoreactive T cells more accurately and to develop a representative index for T1DM development. Besides their use in detection assays, tetramers can also be applied to tolerize autoreactive T cells in T1DM. The observation that high avidity autoreactive T cells bound to HLA class II tetramers can be deleted in in vitro assays implies that HLA class II tetramers could be potent tolerogens in vivo as well. Therefore, the use of HLA class II tetramers for immune intervention strategies in T1DM could be a promising new avenue.
In contrast, development of HLA class 1/epitope tetramers for the detection of CD8+ autoreactive T cells that contribute to TIDM development is limited at the present time. This limitation may be due to the lower degree of associations of HLA class I alleles with the disease compared with the greater number of detected HLA class II associations. Therefore, further studies, the aim of which is to reveal HLA class I allele associations, may contribute to the development of more HLA class I tetramers for use in an immune-based intervention strategy in T1DM.
References
- Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS. Early expression of anti-insulin autoantibodies of humans and the NOD mouse: Evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 2000. 97(4):1701-1706. [DOD] [CrossRef]
- Bingley PJ, Bonifacio E, Mueller PW. Diabetes antibody standardization program: first assay proficiency evaluation. Diabetes 2003. 52:1128-1136. [DOD] [CrossRef]
- Yu L, Eisenbarth GS. Type 1 diabetes, molecular, cellular and clinical immunology. Chapter 10, humoral immunity. Mimeo. www.uchsc.edu/misc/diabetes/oxch10.html. [DOD]
- Borg H, Gottsater A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes 2002. 51(6):1754-1762. [DOD]
- Yamada H, Uchigata Y, Kawasaki E, Matsuura N, Otani T, Sato A, Mutoh K, Kasahara T, Fukushima N, Koike A, et al. Onset age-dependent variations of three islet specific autoantibodies in Japanese IDDM patients. Diabetes Res Clin Pract 1998. 39(3):211-217. [DOD] [CrossRef]
- Hanninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest 1992. 90:1901-1910. [DOD]
- Imagawa A, Hanafusa T, Tamura S, Moriwaki M, Itoh N, Yamamoto K, Iwahashi H, Yamagata K, Waguri M, Nanmo T, et al. Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomenon in type 1 diabetes. Diabetes 2001. 50:1269-1273. [DOD] [CrossRef]
- Peakman M, Tree TI, Endl J, van Endert P, Atkinson MA, Roep BO. Characterization of preparations of GAD65, proinsulin, and the islet tyrosine phosphatase IA-2 for use in detection of autoreactive T-cells in type 1 diabetes: report of phase II of the Second International Immunology of Diabetes Society Workshop for Standardization of T-cell assays in type 1 diabetes. Diabetes 2001. 50(8):1749-1754. [DOD] [CrossRef]
- Karlsson MG, Lawesson SS, Ludvigsson J. Th1-like dominance in high risk first degree relatives of type 1 diabetic patients. Diabetologia 2000. 43:742-749. [DOD] [CrossRef]
- Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, Conlon PJ, Gottieb PA, Putnam AL, Gaur A. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest 2001. 107:173-180. [DOD]
- Kotani R, Nagata M, Moriyama H, Nakayama M, Yamada K, Chowdhury S, Chakrabarty S, Jin Z, Yasuda H, Yokono K. Detection of GAD65-reactive T cells in type 1 diabetics by immunoglobulin-free ELISPOT assays. Diabetes Care 2002. 25(8):1390-1397. [DOD] [CrossRef]
- Liblau RS, Pearson CI, Shokat K, Tisch R, Yang XD, McDevitt HO. High-dose soluble antigen: peripheral T-cell proliferation or apoptosis. Immunol Rev 1994. 142:193-208. [DOD] [CrossRef]
- Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol 2004. 172:5967-5972. [DOD]
- Altman JD, Moss P, Goulder P, Barouch DH, McHeyzer WM, Bell JL, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science 1996. 274:94-96. [DOD] [CrossRef]
- Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther 2005. 12(6):580-591. [DOD] [CrossRef]
- Reijonen H, Kwok WW, Nepom GT. Detection of CD4+ autoreactive T cells in T1D using HLA class II tetramers. Ann N Y Acad Sci 2003. 1005(1):82-87. [DOD] [CrossRef]
- Oling V, Marttila J, Ilonen J, Kwok WW, Nepom G, Knip M, Simell O, Reijonen H. GAD65- and proinsulin-specific CD4+ T-cells detected by MHC class II tetramers in peripheral blood of type 1 diabetes patients and at-risk subjects. J Autoimmun 2005. 25(3):235-243. [DOD] [CrossRef]
- Danke NA, Koelle DM, Cassian Y, Sucheta B, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol 2004. 172:5967-5972. [DOD]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods 1994. 171(1):131-137. [DOD] [CrossRef]
- Mallone R, Kochik SA, Laughlin EM, Gersuk VH, Helena R, Kwok WW, Nepom GT. Differential recognition and activation threshold in human autoreactive GAD-specific T cells. Diabetes 2004. 53:971-977. [DOD] [CrossRef]
- Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci USA 2005. 102(51):18425-18430. [DOD] [CrossRef]
- Panina-Bordignon P, Lang R, van Endert PM, Benazzi E, Felix AM, Pastore RM, Spinas GA, Sinigaglia F. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med 1995. 181:1923-1927. [DOD] [CrossRef]
- Panagiotopoulos C, Qin H, Tan R, Verchere CB. Identification of a beta-cell-specific HLA class I restricted epitope in type 1 diabetes. Diabetes 2003. 52(11):2647-2651. [DOD] [CrossRef]
- Rathmann S, Rajasalu T, Rosinger S, Schlosser M, Eiermann T, Boehm BO, Durinovic-Bello I. Preproinsulin-specific CD8+ T cells secrete IFNgamma in human type 1 diabetes. Ann N Y Acad Sci 2004. 1037(1):22-25. [DOD] [CrossRef]
- Toma A, Haddouk S, Briand JP, Camoin L, Gahery H, Connan F, Dubois-Laforgue D, Caillat-Zucman S, Guillet FG, Carel JC, Muller S, Choppin J, Boitard C. Recognition of a subregion of human proinsulin by class I-restricted T cells in type 1 diabetic patients. Proc Natl Acad Sci USA 2005. 102(30):10581-10586. [DOD] [CrossRef]
- Campanelli R, Palermo B, Garbelli S, Mantovani S, Lucchi P, Necker A, Lantelme E, Giachino C. Human CD8 co-receptor is strictly involved in MHC-peptide tetramer-TCR binding and T cell activation. Internat Immunol 2002. 14(1):39-44. [DOD] [CrossRef]
- Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type 1 diabetes. J Clin Invest 2002. 109:131-140. [DOD] [CrossRef]
- Oikawa Y, Shimada A, Yamada S, Motohashi Y, Nakagawa Y, Irie J, Maruyama T, Saruta T. High frequency of Valpha24(+) Vbeta11(+) T-cells observed in type 1 diabetes. Diabetes Care 2002. 25(10):1818-1823. [DOD] [CrossRef]
- Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, Strominger JL, Hafler DA. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature 1998. 391(6663):177-181. [DOD] [CrossRef]
- Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest 2002. 110(6):793-800. [DOD] [CrossRef]
|